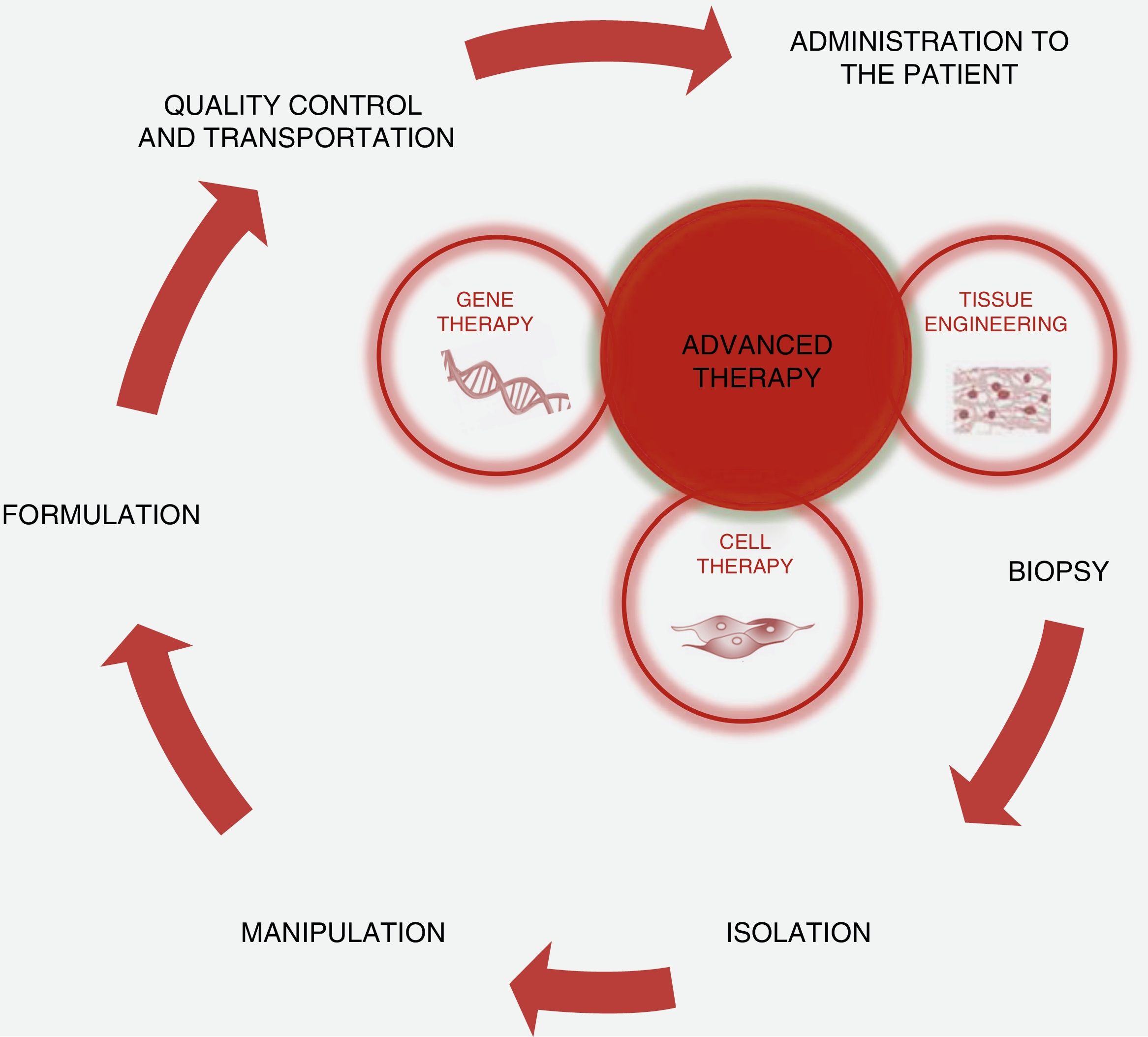
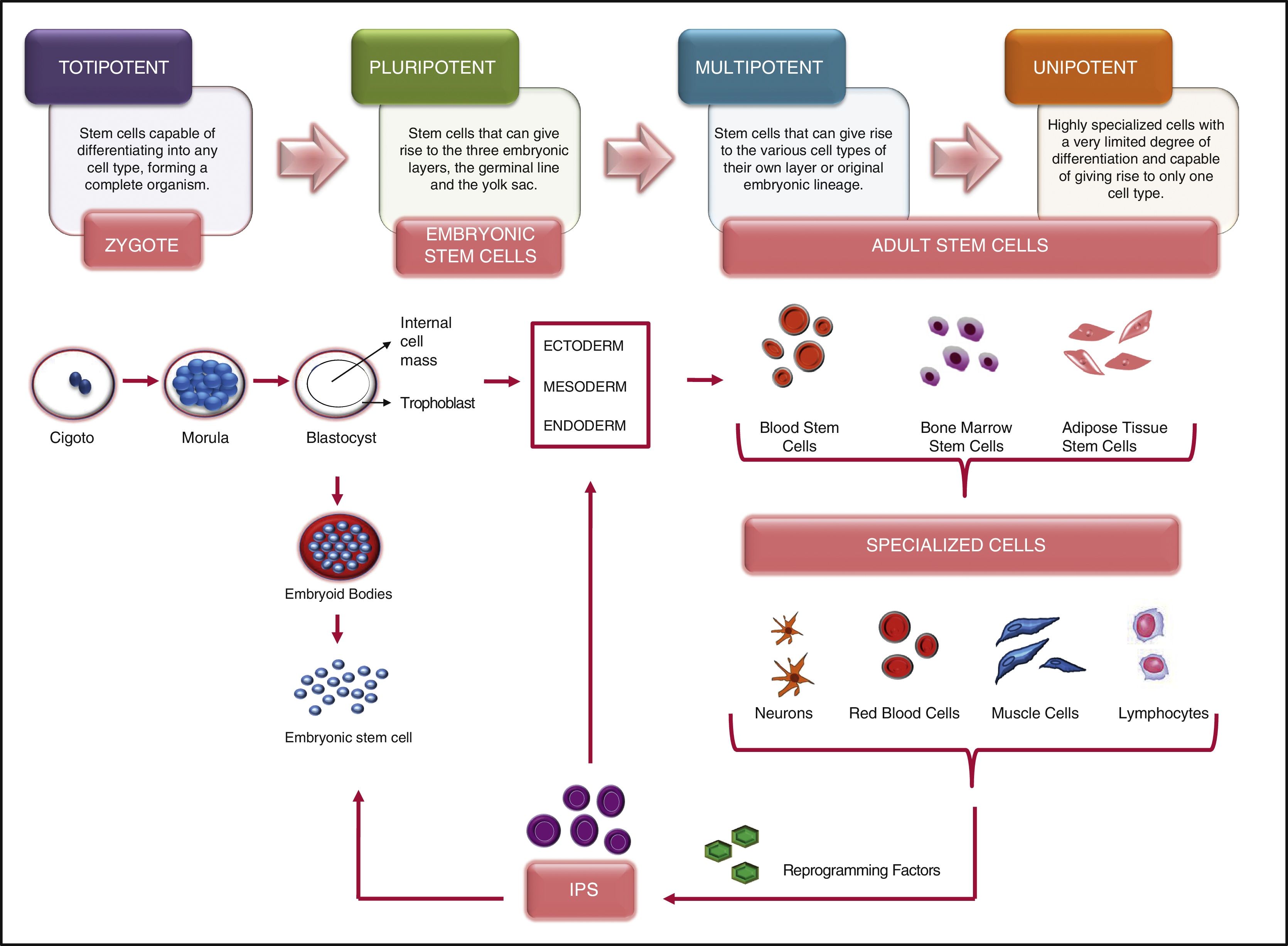
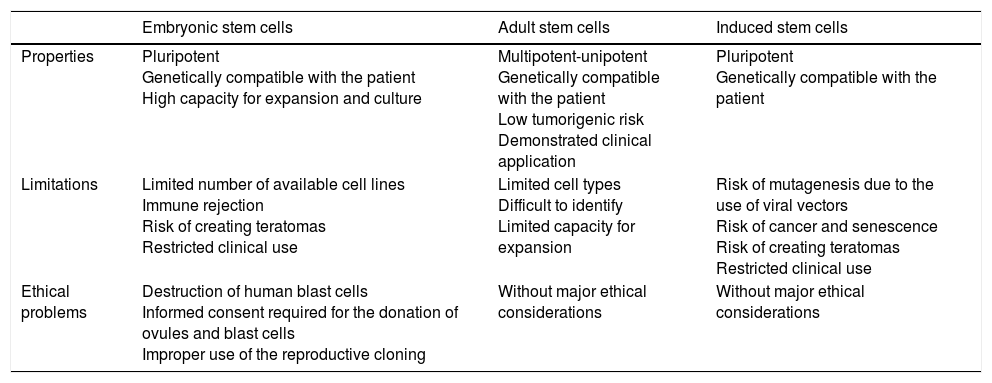
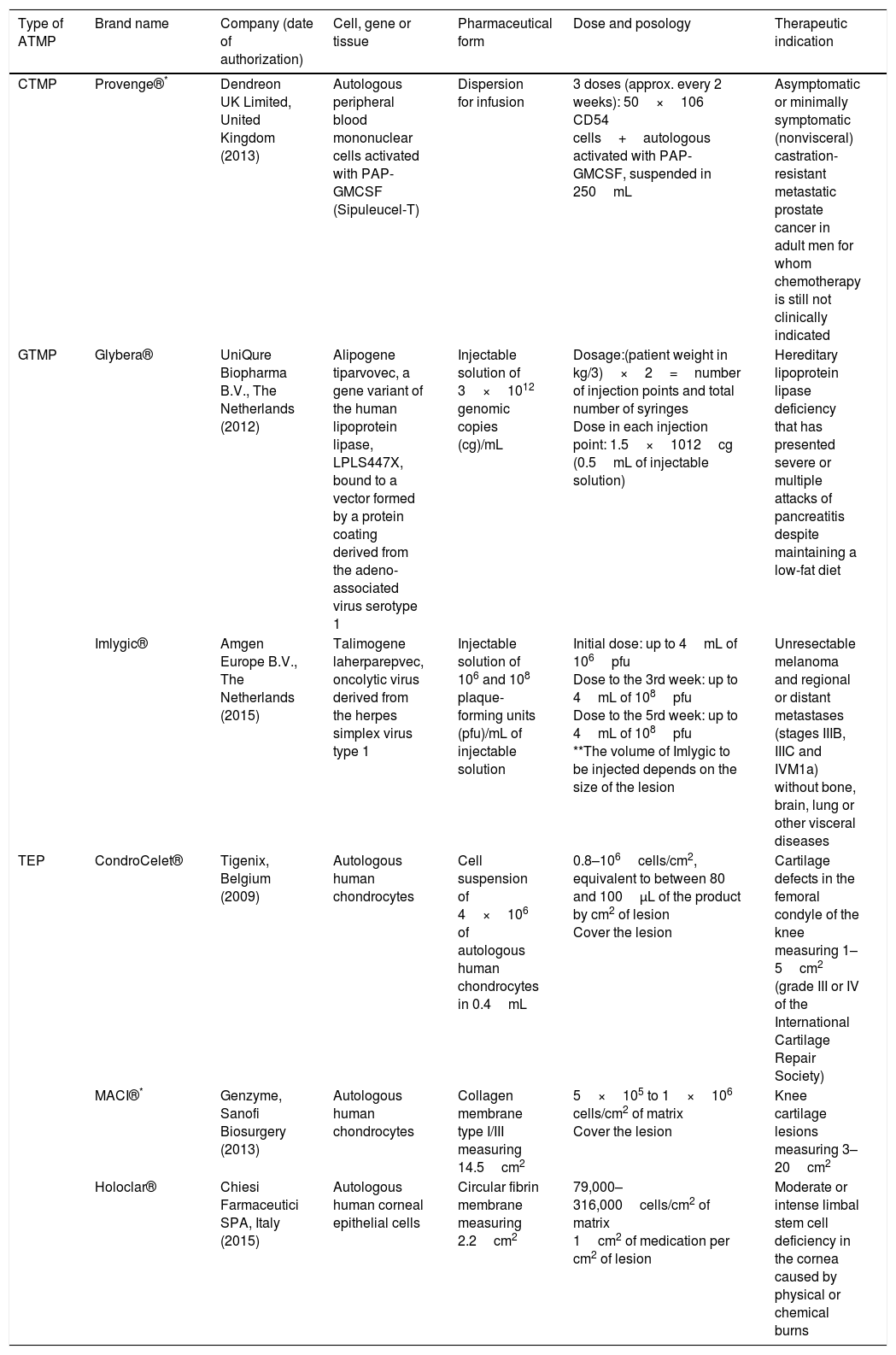
Scientific and technical advances in the areas of biomedicine and regenerative medicine have enabled the development of new treatments known as “advanced therapies”, which encompass cell therapy, genetics and tissue engineering. The biologic products that can be manufactured from these elements are classified from the standpoint of the Spanish Agency of Medication and Health Products in advanced drug therapies, blood products and transplants. This review seeks to provide scientific and administrative information for clinicians on the use of these biologic resources.
Los avances científicos y técnicos en el área biomédica y la medicina regenerativa han permitido el desarrollo de nuevos tratamientos, denominados «terapias avanzadas», que engloban la terapia celular, la génica y la ingeniería tisular. Los productos biológicos que pueden fabricarse a partir de estos elementos se clasifican desde el punto de vista de la Agencia Española del Medicamento y Productos Sanitarios en medicamentos de terapias avanzadas, productos derivados de la sangre y trasplantes. Esta revisión pretende aportar información científica y administrativa, de utilidad para el clínico, sobre el uso de estos recursos biológicos.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.
Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<