This work is a review of the scientific evidence on the oral treatment of adult patients with Gaucher disease type 1 (GD1) with a clinical guideline format according to the Agree II regulations. It describes the main differences between the two oral treatments currently available for treating this disease (miglustat and eliglustat).
This review reminds us that the criteria for starting oral treatment in patients with GD1 must be assessed individually. Although miglustat and eliglustat are both glucosylceramide synthase (GCS) enzyme inhibitors, they have different mechanisms of action and pharmacological properties and should never be considered equivalent. Miglustat is indicated in patients with non-severe GD1 who cannot receive other first-line treatments, while eliglustat is indicated as first-line treatment for patients with GD1 of any severity without the need for prior stabilization with enzyme replacement therapy (ERT). It is important to emphasize that in order to start treatment with eliglustat, we must know the CYP2D6 metabolic phenotype and its association with drugs metabolized through the CYP2D6 and CYP3A4 cytochromes–or alternatively those that use P-Glycoprotein must be evaluated on an individual basis. During pregnancy, the use of eliglustat should be avoided; only ERT can be used. Unlike miglustat, whose adverse effects have limited its use, eliglustat has not only demonstrated similar efficacy to ERT but has also been shown to improve the quality of life of patients with GD1.
Revisión de la evidencia científica sobre el tratamiento oral de pacientes adultos con enfermedad de Gaucher tipo 1 (EG1), con formato de guía clínica, según la normativa Agree II. Se describen las principales diferencias entre los dos tratamientos orales disponibles actualmente para el tratamiento de esta entidad (miglustat y eliglustat).
En esta revisión se recuerda que los criterios para iniciar el tratamiento oral en los pacientes con EG1 deben valorarse de forma individualizada. Si bien miglustat y eliglustat son inhibidores de la enzima glucosilceramida sintetasa (GCst), los dos presentan diferentes mecanismos de acción y propiedades farmacológicas y nunca se deben considerar como equivalentes. Miglustat está indicado en pacientes con EG1 no grave que no pueden recibir otro tratamiento de primera línea, mientras que eliglustat está indicado en pacientes con EG1 con cualquier gravedad, en primera línea y sin necesidad de estabilización previa con tratamiento de reemplazo enzimático (TRE). Es importante enfatizar que para iniciar tratamiento con eliglustat debemos conocer el fenotipo metabólico CYP2D6 y que su asociación con fármacos metabolizados a través de los citocromos CYP2D6 y CYP3A4 –o bien que utilicen la glicoproteína P– se debe evaluar individualmente. Durante el embarazo se debe evitar el uso de eliglustat, pudiéndose emplear únicamente el TRE. A diferencia de miglustat, cuyos efectos adversos han limitado su utilización, eliglustat no solo ha demostrado una eficacia similar a la TRE, sino que ha demostrado mejoría en la calidad de vida de los pacientes EG1.
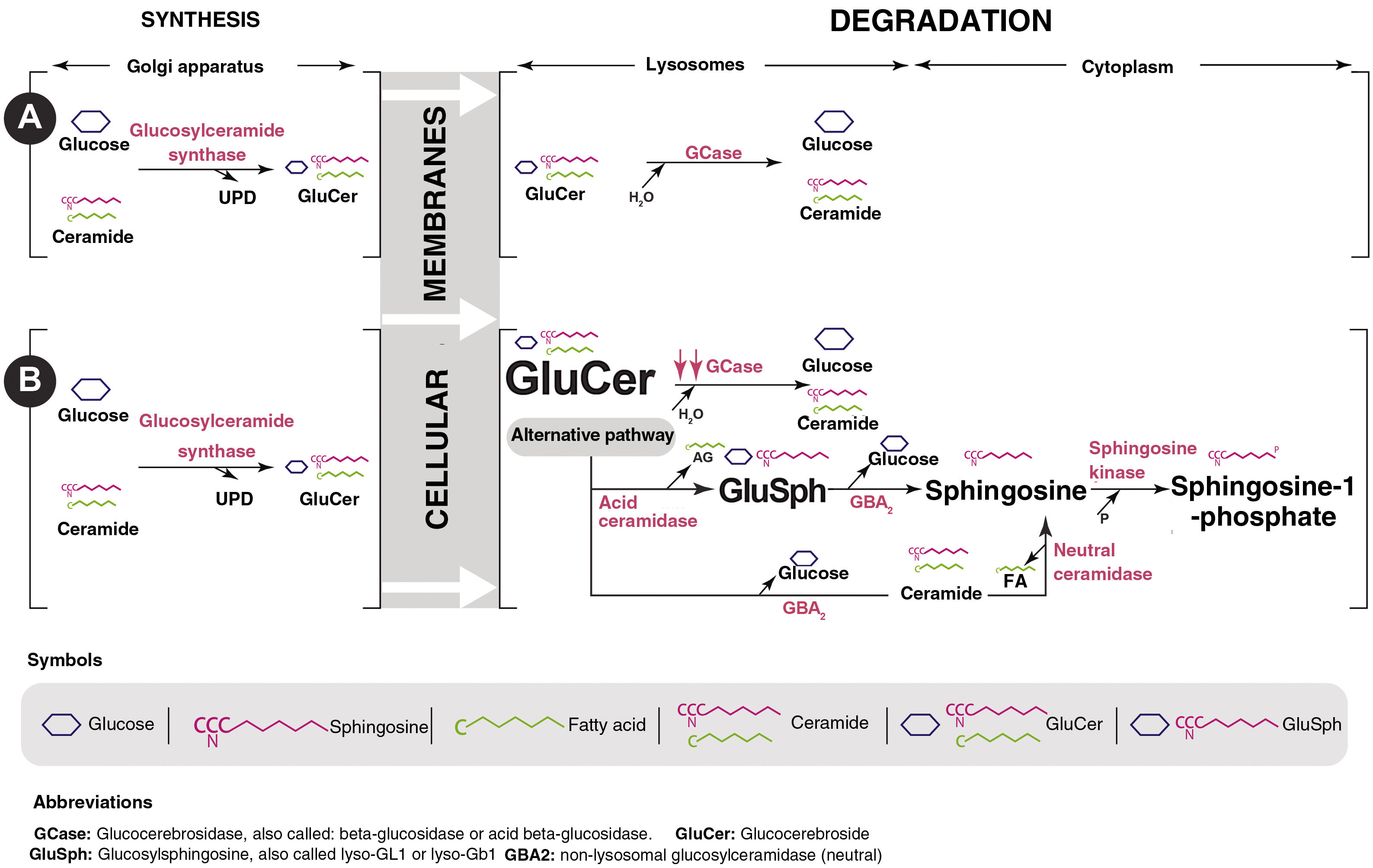
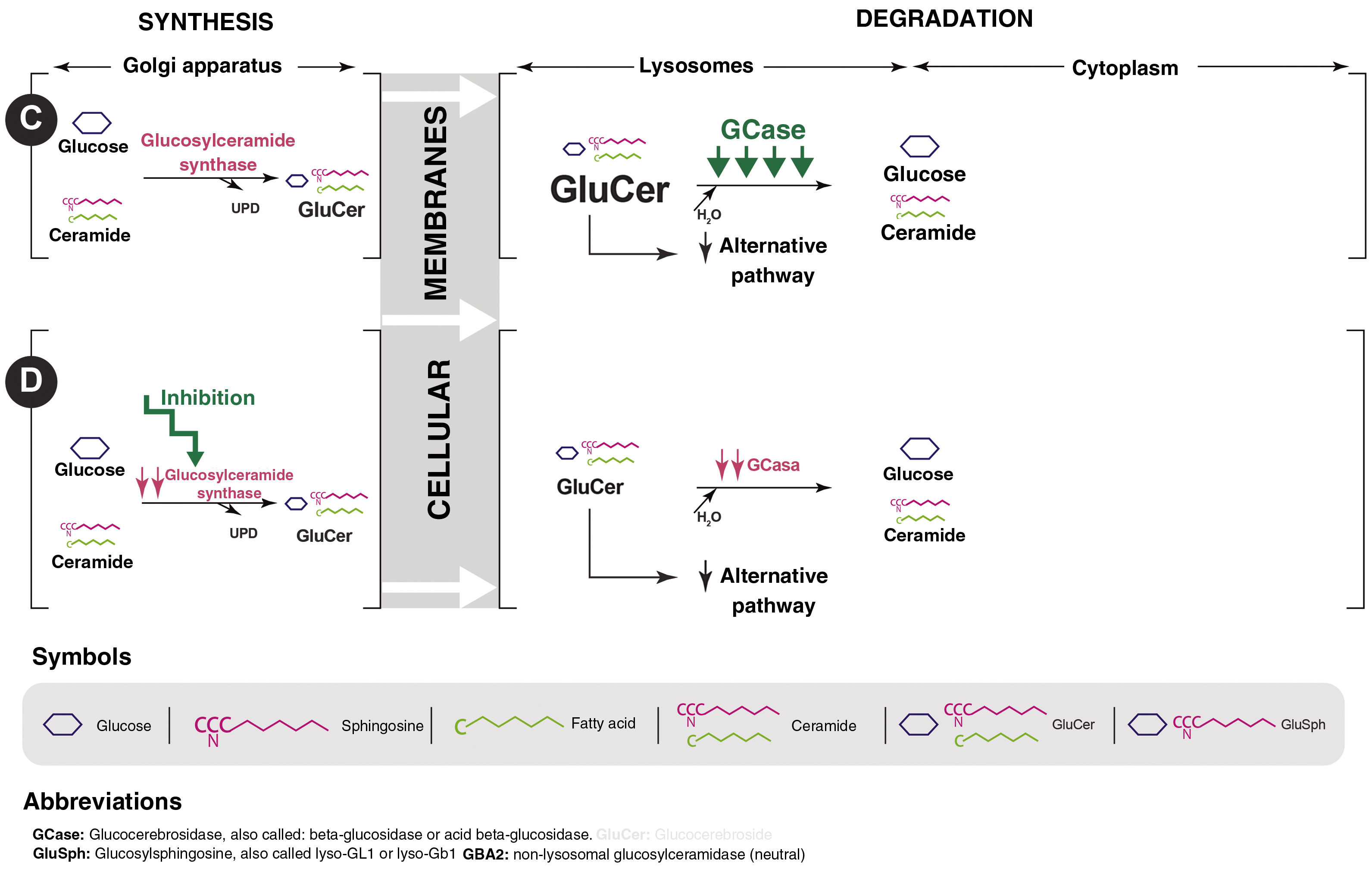
Gaucher disease (GD) is a lysosomal storage disease of autosomal recessive inheritance caused by mutations in the GBA gene that lead to a defect in the synthesis or functional abnormality of the glucocerebrosidase (GCase) protein. This enzyme deficiency produces an accumulation of its substrate, glucosylceramide or glucocerebroside (GluCer), in the lysosome which, in turn, is deacylated, giving rise to glucosylsphingosine (GluSph or lyso-GL1) and later to sphingosine-1-phosphate (Fig. 1). They are partly responsible for the symptoms and complications of the disease.1,2
The type 1 variant (GD1), or non-neuropathic variant, is the most common in our setting. It has an approximate annual incidence of one case out of every 50,000 live births and an estimated prevalence of 1.5 cases/100,000 inhabitants.1,2 GD1 is characterized by the onset of splenomegaly, thrombocytopenia (or other cytopenia), hepatomegaly, bone pain or necrosis, distal femur deformity, elevation of inflammatory markers (ferritin, tartrate-resistant acid phosphatase, or angiotensin-converting enzyme), polyclonal/monoclonal hypergammaglobulinemia, and mucocutaneous hemorrhage in varying proportions.1,2
Since about 30 years ago, treatment has been based on the administration of enzyme replacement therapy (ERT), which has led to spectacular improvements in all aspects related to these patients. At the beginning of this century, oral therapy with substrate synthesis inhibitors (miglustat) was introduced. Oral therapy has undergone a substantial change in recent years with the availability of eliglustat. This drug has demonstrated an efficacy similar to ERT and has changed the management of these patients. However, a certain degree of confusion remains regarding the indications and efficacy of the two currently available GluCer synthase inhibitor drugs: miglustat and eliglustat.
The main objective of these guidelines is to update and standardize oral treatment for adult patients with GD1, establishing the differences between eliglustat and the previously existing oral treatment, miglustat. Given that the two available drugs act by inhibiting glucosylceramide synthase (GCst), we considered it necessary to clarify differences regarding their efficacy and indication in the treatment of adult patients with GD1. This includes patients without previous treatment and those previously treated with enzymatic therapy with or without associated comorbidities.
Materials and methodsIn order to develop this document, a working group on GD in adults was created. It comprised five specialists from the hematology, hemotherapy, and internal medicine departments of five Spanish hospitals who had proven experience in the diagnosis and treatment of these patients as well as in the development of clinical practice guidelines.
Regarding patients’ points of view and preferences, there was no direct participation from patients in the development of this document, but information from the literature that addresses this issue was used.
This document is aimed at physicians in any medical specialty who would like more information on the indications and management of oral treatment for patients with GD1 as well as other healthcare personnel involved. In the creation this document, the issues shown in Table 1 were answered.
Questions that must be answered in substrate reduction therapy in adult patients with GD1.
| 1. Have oral treatments demonstrated efficacy, effectiveness, efficiency, and safety? |
| 2. Do oral treatments have the same effectiveness as ERT? |
| 3. Are eliglustat and miglustat equally equivalent in efficacy, effectiveness, efficiency, and safety? |
| 4. Do eliglustat and miglustat have the same indication? |
| 5. What is the dose and instructions for administration? |
| 6. Can it be used in children? |
| 7. Can it be used during pregnancy and breastfeeding? |
| 8. Can it be used in polymedicated patients? |
| 9. Can it be used in patients with heart disease, liver disease, or kidney disease? |
| 10. Is eliglustat indicated in previously untreated adult patients with GD1 or patients treated with ERT? |
| 11. Is SRT indicated in severe patients? |
| 12. Is it necessary to stabilize a patient without previous treatment with ERT before starting SRT? |
| 13. Do disease biomarkers improve? |
| 14. Are they capable of stabilizing, improving, reverting, or preventing bone, hematologic, and/or organ complications in a similar manner? |
| 15. Do eliglustat and/or miglustat improve quality of life? |
GD1, Gaucher disease type 1; ERT, enzymatic replacement treatment; SRT, substrate reduction therapy.
The target population this document is aimed at is male or female patients older than 18 years of age with GD1 who need to begin treatment for their disease or who prefer or need to change to oral treatment for their disease. Exceptions are patients with GD1 who are younger than 18 years of age, women who are pregnant or wish to become pregnant soon, men who wish to have children soon, those whose CYP2D6 metabolizer genotype contraindicates the use of treatment with GCst inhibitors, those who have comorbidities or require drugs which contraindicate the use of GCst inhibitors, or those with any of the other types of GD.
No pilot studies have been conducted in Spain on any of the oral treatments. There are approximately 300 patients with GD1 in our country, of which 39 have been treated with miglustat. A significant percentage of them had to abandon treatment due to its side effects.3 From 2017 until now, about 100 patients have been treated with eliglustat without any alarm symptoms or symptoms related to patient safety being reported in Spain, according to a consultation of the Spanish Agency of Medicines and Medical Devices website using the search term “eliglustat” (search conducted in June 2021: https://www.aemps.gob.es/tag/seguridad-8 and https://www.aemps.gob.es/acciones-informativas/notas-informativas-medicamentos-de-uso-humano).
To achieve this aim, it was decided to evaluate the two oral medications available for the treatment of adult patients with GD1 (miglustat and eliglustat) in each of the following areas: mechanisms of action, evidence on effectiveness, indications and contraindications, follow-up, and tolerability. The work method followed was that articles published on oral GD1 treatment were identified by means of a search conducted between February and May 2021 on Embase, the Cochrane Library, and MEDLINE, as were the technical datasheets of the two drugs to be analyzed. Using the keywords “eliglustat” and “miglustat,” 134 articles were identified in MEDLINE, 16 in the Cochrane Library, and 32 in Embase.
The selection and later classification of the articles to be reviewed was done according to the level of evidence. Level 1 included systematic reviews of randomized clinical trials (CTs). Level 2 included well-designed randomized CTs. Level 3 included nonrandomized CTs, cohort studies, case-control studies, and time series with a control group. Lastly, level 4 included case series. Due to the idiosyncrasy of GD1, which is classified as an ultrarare disease, the degree of recommendation was categorized according to the experts’ clinical judgment and experience. In total, 61 references were found, which constitute this document’s bibliography. For the recommendations, the degrees of evidence and levels of recommendation of the Scottish Intercollegiate Guidelines Network (SIGN)4 were used.
In subsequent meetings, the selected evidence was presented in order to debate the recommendations gathered. Attendees voted in order to establish the degree of consensus for each of the recommendations. Recommendations were considered accepted when at least three of the five authors were in agreement.
Two external evaluators—hematologists/internal medicine specialists who are experts in GD1—were invited to review the methodological rigor by means of the Agree II instrument. The external reviewers’ considerations were used to focus on the methodological aspects and the clarity of the recommendations.
This publication has received scientific endorsement from the Spanish Society of Hematology and Hemotherapy (SEHH, for its initials in Spanish) and the Spanish Society of Internal Medicine (SEMI, for its initials in Spanish).
ResultsTreatment of Gaucher disease type 1Historically, GD1 was treated with surgery, such as a splenectomy or orthopedic procedures. New treatments have significantly changed the natural history of this disease, although curative treatment still does not exist.5
The criteria for starting treatment in patients with GD1 must be evaluated individually. Starting treatment is usually recommended in patients with symptomatic disease, including pain—either abdominal (visceromegaly) or bone—liver impairment, lung disease, fatigue, limitations for exercise, weakness and cachexia, any skeletal manifestation (including radiological abnormalities, such as Erlenmeyer flask abnormality), thrombocytopenia (generally less than 60,000 platelets/mm3, decreasing figures, or cases with hemorrhagic complications regardless of the platelet count), hemoglobin levels 2 g/dL below the normal limit for the patient’s age and sex, and in general in all cases in which a deterioration in quality of life or a progression of symptoms are observed. In asymptomatic patients, the indication for treatment is conditioned by the severity of bone involvement.6,7
The general short- and long-term objectives7,8 are:
- 1)
Normalize hemoglobin levels, eliminating dependence on transfusions.
- 2)
Increase the platelet count to levels above 100,000 platelets/mm3 and reduce the tendency to hemorrhage.
- 3)
Reduce visceromegaly and prevent hepatic fibrosis, cirrhosis, and portal hypertension.
- 4)
Prevent or improve lung disease (pulmonary hypertension, hepatopulmonary syndrome).
- 5)
Prevent bone complications such as osteopenia, osteoporosis, avascular necrosis, bone crises, and pathological fractures. In addition, reduce or eliminate chronic analgesia due to bone pain.
- 6)
Maintain good quality of life, promote optimal growth, and normalize life expectancy.
- 7)
In pregnancy, prevent complications during the pregnancy, birth, and postpartum period.
At present, there are two types of pharmacological treatment approved for GD1 (Fig. 2). The first treatment type is ERT, which was first approved for patients with GD1 at the beginning of the 1990s. The first drug used was alglucerase (purified placental GCase), which was later substituted by imiglucerase. Later, velaglucerase alfa was approved in 2009 and taliglucerase alfa in 2011.2,9,10 In Spain, only imiglucerase and velaglucerase alfa are commercially available.
The initial dose depends on the patient’s severity at diagnosis: severe patients or those with rapidly progressing disease and comorbidities should start with 60 U/kg every two weeks. Severe patients or those with rapidly progressing disease should start with 30–60 U/kg every two weeks. Mild patients or those with moderate disease should start with 15–30 U/kg every two weeks.6,11 The maintenance dose is calculated after at least one year of initial therapy and modified according to the results obtained.6
ERT has been demonstrated to improve all clinical aspects and biological parameters of patients with GD1. The main disadvantages of this therapy are its intravenous administration, the patient’s dependence on the hospital, the possibility of the patient developing an aversion to needles, and the need administer the infusion in a hospital setting at first—with the increase in additional costs this entails10—though it is possible to administer it at home if conditions are met.
The second treatment type is substrate reduction therapy, approved at the beginning of the 2000s. The first drug was miglustat. Later, eliglustat was approved in 2015. Both have shown beneficial effects in GD1, but with significant differences in both the mechanisms of action, the objectives achieved, and the possible side effects that one or the other cause. These will be explained in this guide.
Other treatments are currently in development, including molecular chaperones or genetic therapy, among others, in addition to supportive therapy; these will not be discussed in these guidelines.
Mechanisms of actionOral treatment for GD1 is based on the use of substrate reduction drugs, a strategy used in lysosomal diseases since it was first described by Radin et al. in 197212 and used specifically in GD in 1996.13 These drugs reduce GCst activity, which catalyzes the synthesis of GluCer from UDP-glucose and ceramide. The inhibition of this first step in the production of glycosphingolipids decreases the production of GluCer, which is the substrate for the deficient enzyme in GD. In addition, it allows for the minimal quantity that is produced to be degraded by the residual GCase present in all patients with GD1, eliminating the pathogenic effects of GluCer accumulation and its main byproduct, lyso-GL114 (Fig. 1). There are currently two drugs that inhibit GCst. They have different mechanisms of action and pharmacological properties, which explains the differences in their efficacy.
Miglustat is an iminosugar byproduct (N-butyldeoxynojirimycin) analog of D-glucose that is water-soluble. It was initially developed as an α-glucosidase inhibitor for treating HIV infection. It was later observed that it was a reversible GCst inhibitor in the intermediate micromolar range.15 Its IC50 (concentration which causes 50% inhibition) is 20 µl/L.16
Its main disadvantage is that it is a nonspecific inhibitor of α-glucosidases i and ii and intestinal disaccharides (lactase, sucrase, and maltase), which leads to the onset of undesirable adverse effects. After the administration of the therapeutic dose of 100 mg/8 h, it is absorbed and reaches its peak plasma concentration of 0.86 µg/mL in 2.5 h, which decreases modestly if it is administered with food. It circulates without binding to proteins with a distribution volume of 83 L.
It is mainly eliminated renally after a half-life of six to seven hours; its elimination is reduced in patients with kidney failure. Although miglustat passes the blood-brain barrier (MW 219.28 g/mol, water soluble), it does not have an effect on the neurological symptoms of patients with GD that is demonstrable in CTs.16
Eliglustat tartrate (Genz-112638) is a ceramide analog with a structure similar to D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol. Its inhibitory activity is reversible, potent, and selective for GCst with an IC50 in the nanomolar range (10 ng/mL, 24 nM).14,17–19 It does not inhibit the function of intestinal disaccharides. Eliglustat has a low oral bioavailability (<5%) because it undergoes an important hepatic first pass effect as it is metabolized by the hepatic cytochrome CYP2D6 and, to a lesser extent, by the cytochrome CYP3A4. This conditions its pharmacokinetics according to patients’ CYP2D6 metabolizer phenotype. In this regard, for extensive metabolizers (EM) or intermediate metabolizers (IM), eliglustat has a pharmacokinetic profile that is non-linear, time-dependent, and greater than the dose-dependent pharmacokinetics. On the contrary, poor metabolizers (PM) follow a linear and time-independent pharmacokinetic profile. The therapeutic dose is adjusted according to the patient’s metabolizer phenotype.
After administration of the therapeutic dose, it is absorbed and reaches its peak plasma concentration of 12.1−25 ng/mL (EM) or 113−137 ng/mL (PM) at 1.5 h (EM) or 3 h (PM).17,18 Its absorption does not vary with food, although it decreases slightly after intake of food rich in fat. It circulates in plasma bound to proteins (76%–83%). Its distribution volume is very high (835 L), which ensures widespread tissue distribution.
It is eliminated through feces (51%) and urine (42%) after a half-life that varies from 6.5 h (EM) to 8.9 h (PM), depending on the degree of metabolization.17–19 Despite its low molecular weight (MW 404.5 g/mL), eliglustat does not cross the blood-brain barrier because it is a substrate of P-glycoprotein.14,17
EvidenceMiglustatBased on data from CTs,20–22 miglustat was approved in the European Union in 2002 and in the USA in 2003. Studies performed in adults with GD1 without previous treatment and with mild or moderate forms showed effective results in the reduction of spleen and liver volumes and in an increase in hemoglobin and platelet levels during the 12–36 months of treatment.23,24 An improvement in bone mineral density in both cancellous and cortical bone was also shown.25 However, as the patients included in the studies had mild and moderate disease and thus little bone involvement, it is not possible to draw conclusions regarding the clinical response in bone with this treatment. Therefore, it was concluded that miglustat could serve as maintenance therapy in some patients who were previously stabilized with ERT but who for some reason can no longer take it.
However, later studies showed that a group of patients who changed to miglustat after being stabilized with ERT showed an increase in chitotriosidase and a decrease in hemoglobin and platelets, which indicated disease progression.20,24,26 Furthermore, after the second year of miglustat monotherapy, 26% of patients abandoned treatment due to adverse events or disease progression.3
Nevertheless, the main problems with the drug are its side effects, especially gastrointestinal side effects such as diarrhea and flatulence, which are the main cause for abandoning treatment.25 Other important side effects are weight loss, tremors, and peripheral neuropathy as well as neurological disorders that affect up to 29% of patients.27 For all of these reasons, treatment abandonment is very high and although it varies greatly according to country (13% in Spain versus 68% and 69% in France and the United Kingdom, respectively), nearly 50% of patients stop treatment.3
Therefore, miglustat is an iminosugar indicated as second-line treatment in patients with mild or moderate GD1 who, for various reasons, cannot be treated with ERT or eliglustat. It is not advised for any newly diagnosed to patient start therapy with miglustat,28 it should only be maintained in the few cases of patients with GD1 who tolerate the treatment well and have continued with it for years.3
EliglustatThe search method used allowed for selecting the controlled CTs listed below.
Efficacy studiesPhase II clinical trials (NCT00358150). The fundamental trial for evaluating the efficacy of eliglustat in GD1 was conducted between 2010 and 2019 in 26 adults who were previously untreated. In the 19 patients who completed the eight years of follow-up, the study found significant improvements in visceromegaly (reduction in the spleen and liver of 69% and 34%, respectively), hematological parameters (a 2.2 g/dL increase in hemoglobin and 113% increase in platelets), classic biomarkers and lyso-GL1, and quality of life as well as normalization of lumbar spine T-scores which previously showed osteopenia.29–32
Phase III clinical trials. Three fundamental randomized, multicenter, multinational clinical trials have been published:
ENGAGE (NCT00891202)- -
This was a double-blind, placebo-controlled trial in 40 patients with GD1 without prior treatment who were randomized 1:1 into treatment with eliglustat or a placebo for nine months. All patients had splenomegaly with thrombocytopenia and/or anemia. The primary outcome measure was the percent change in spleen volume. Hematological parameters were evaluated as secondary efficacy criteria. Improvement in biomarkers and bone parameters were established as tertiary criteria.
- -
At nine months, a significant improvement was demonstrated in hemoglobin (+0.69 g/dL vs. − 0.54 g/dL) and platelet counts (+32% vs. − 9%) as well as liver (−5.2% vs. + 1.4%) and spleen size (−27.8% vs. + 2.3%), which decreased with eliglustat, but not with the placebo. In the eliglustat group, statistically significant improvements were also observed in the bone marrow burden and in biomarkers (p = 0.0021), which was not observed with the placebo.33 In a second extension of the trial, continuation of treatment with eliglustat for nine more months resulted in a significant improvement in all disease parameters and subjects who had previously received the placebo saw significant improvements in spleen and liver volumes, hemoglobin, and platelets. Treatment with eliglustat was also associated with improvements in the bone marrow burden score, bone mineral density, and biomarkers.34
- -
This trial evaluated the efficacy and safety of eliglustat in terms of noninferiority to imiglucerase in stabilized patients. In the 106 patients out of 159 patients with GD1 who changed to eliglustat or maintained ERT for 12 months, all clinical characteristics and hematological parameters were stable in both groups. Likewise, the bone marrow burden did not change in this period of time.35
- -
In the long-term extension of this study, after one year, all patients continued with or changed to eliglustat and it was demonstrated that liver and spleen size as well as hemoglobin and platelet counts remained stable over one to four years of treatment. In this extension, significant increases were also shown in the lumbar spine (p < 0.0001 during the four years) and femoral Z-scores (p = 0.005 in the first year and p = 0.02 in the third).36
- -
This was a double-blind comparator trial for the dosing of once-daily eliglustat with an approved regimen of two times per day in adults with GD1 for a median of 3.3 years. It was demonstrated that patients who received eliglustat two times per day showed greater overall stability.37
The Gaucher Registry from the International Collaborative Gaucher Group (NCT00358943). Very few publications on eliglustat outside of clinical trials have enough cases to allow for drawing conclusions.
In 2020, Mistry et al.38 published a study on 231 patients (89% from the USA) with GD1 from the international registry. Patients were divided into three groups: 19 new patients and 212 who changed from ERT, of which 36 were splenectomized and 176 were nonsplenectomized. All had received eliglustat for a mean of two years (minimum one year) and results were obtained at the beginning and the end of the study. In all new patients, statistically significant clinical improvements were observed in hematological parameters and visceromegaly. In patients who changed from ERT, stabilization or a small improvement was observed and all patients were within the therapeutic objectives established for ERT.
Chitotriosidase values also decreased significantly, which indicates a regression in inflammation and the polarization of aberrant macrophages which underlie this disease's pathophysiology. The bone evaluation was limited by the short study period, but in nonsplenectomized patients who changed from ERT, a significant increase was observed in the lumbar spine Z-score after treatment with eliglustat.
These data corroborate the results obtained in pivotal clinical trials. The best results were obtained in patients who had worse data at the start of treatment. Eliglustat was very well tolerated. Treatment was discontinued in 9% (22/231) of patients for different reasons, such as adverse effects, preference for enzymatic treatment, lack of availability of the drug or it not being covered by medical insurance, and, in seven cases, due to the intention to become pregnant.
More limited evidence found in a retrospective multicenter study of 14 patients in treatment with eliglustat. Regarding the behavior of the lyso-GL1 and chitotriosidase biomarkers in patients treated with eliglustat versus those treated with enzymes currently available on the market, better behavior was observed in patients in the eliglustat group in terms of a statistically significant reduction in biomarkers associated with GD.39
Studies on toxicity, pharmacokinetics, or side effects- -
The grouped analysis of adverse events by Peterschmitt found that among 393 patients with GD1 treated with eliglustat in four clinical trials (NCT00358150, NCT00891202, NCT00943111, and NCT01074944), there were no severe adverse events or interruptions of the drug due to elevation of ALT levels or acute liver failure.40 The follow-up study conducted by the same authors evaluated the toxicity or side effects of eliglustat in the four aforementioned works, representing an exposure of 1400 patient-years to the aforementioned drug and with a mean treatment duration of 3.6 years (maximum: 9.3 years). Eighty-one percent of patients remained in their respective trial until the commercial availability of eliglustat or until the end of the trial, nine patients (2.3%) withdrew due to one or more reported adverse events related to eliglustat, and all adverse events except one were mild or moderate. In general, 97% of the adverse events were mild or moderate and the investigator reported that 86% were not related to eliglustat. The general rate of adverse events decreased over time and did not correlate to the drug dose.41
- -
A study on the impact of liver and kidney impairment on tolerability and pharmacokinetics in patients with GD1 treated with eliglustat analyzed data from phase I studies (NCT02536937/NCT02536911) after a single 84-mg dose of eliglustat. EM patients with moderate liver failure (LF) or severe kidney failure presented with greater plasma concentrations of eliglustat. Higher exposures of eliglustat were also predicted in EM patients with mild LF after coadministration with a CYP2D6 or CYP3A4 inhibitor in repeated doses.42
- -
Effect of eliglustat on the pharmacokinetics of digoxin (P-glycoprotein substrate), metoprolol (CYP2D6 substrate), and oral contraceptives (CYP3A substrate) and the absorption of eliglustat when it is coadministered with gastric acid secretion inhibitors: healthy subjects were enrolled in four phase I clinical trials, which verified that eliglustat was well tolerated and, when coadministered with drugs that are a P-glycoprotein or CYP2D6 substrate, lower doses of these drugs are required. On the other hand, eliglustat can be coadministered with oral contraceptives and gastric acid secretion inhibitors without modifying the dose.43
In addition to what has already been discussed regarding the ENGAGE and ENCORE trials, one publication specifically focused on bone evaluation:
- -
The skeletal effects of eliglustat were evaluated by means of prospective monitoring of bone mineral density, the development of fractures, marrow infiltration by Gaucher cells, and the onset of focal bone lesions and infarction during the phase II trial NCT00358150. The study showed that lumbar spine bone mineral density increased significantly (p = 0.02; n = 15) by a mean of 9.9% (14.2% of patients) since the start until year 4. Lumbar spine T-scores also increased significantly (p = 0.01) from a mean of −1.6 (1.1) to −0.9 (1.3). Femoral T-scores remained normal for four years. Magnetic resonance imaging (MRI) of the femur showed that 10/18 (56%) patients had less bone marrow infiltration compared to the initial images and one patient with early bone improvement had transitory worsening in year 4. No fractures or bone crises were described.
At the start of the study, 8/19 (42%) patients had focal bone lesions, which remained stable, and 7/19 (37%) patients had bone infarcts, which improved in one patient in year 2. In year 4, a previously inexistent bone lesion was discovered; it was asymptomatic, indeterminate, and later resolved.31
Post-hoc analysisApart from ENCORE,36 two other post-hoc analyses compared clinical stability between the use of imiglucerase or velaglucerase and eliglustat.
- -
Ibrahim et al. compared the response to eliglustat in patients with previously untreated GD1 in phase II and III clinical trials (ENGAGE) with the response to imiglucerase in another cohort of patients without previous treatment who belonged to the International Collaborative Gaucher Group. The visceral and hematological parameters improved significantly in both groups in a similar manner, without including effects on the bone or any data being collected on possible adverse effects.44
- -
Pleat et al. conducted a subanalysis of the ENCORE study which compared stability in adult patients with GD1 who changed from velaglucerase to imiglucerase or eliglustat: 88% of patients who changed to imiglucerase and 90% of those who changed to eliglustat reached the trial's outcomes.45
There is one study with the aim of comparing the efficacy of imiglucerase with eliglustat in the treatment of patients with GD. In that study, searches were conducted on PubMed/MEDLINE, the Cochrane Library, Scopus, Web of Science, Embase, and Google Scholar up to August 2018 and all randomized, quasi-randomized controlled, and cohort studies on patients with GD1 which compared imiglucerase to eliglustat were included.
The results showed no significant differences between the two drugs in terms of an increase in hemoglobin in blood, platelet count, or a reduction in liver or spleen size. The findings of this review showed that both medications are effective in the treatment of GD1 and there are no statistically significant differences between their efficacies.46
Study on the financial impactNalysnyk et al. conducted a study on the financial impact on the US health system associated with patients who transition from receiving ERT to receiving eliglustat for the treatment of adults with GD1. The annual costs of ERT were calculated assuming a bimonthly dose of 47.4 U/kg, for example in a patient who weighs 72 kg who receives 24 infusions per year. The results of the study demonstrated that a greater use of orally administered eliglustat—instead of the more expensive treatment based on enzymatic infusion—led to significant savings.47
Indications/contraindicationsThe two oral GCst inhibitor drugs, miglustat and eliglustat, have different pharmacokinetics and effectiveness, which leads to clearly different indications and warnings.
Miglustat is indicated for adult patients with mild to moderate GD1 in which ERT is not appropriate. The criteria of mild or moderate disease is arbitrary, but would include patients with hemoglobin levels greater than 9 g/dL, platelet counts greater than 50 × 109/L, and those without evidence of progressive bone disease.15 It does not provide any advantages over ERT, as it is less effective28; its only advantage is that it is an orally administered drug.23 Despite the fact that its concomitant administration with ERT has been evaluated in patients with an insufficient response to maximum doses,15 this regimen must not be used outside of clinical trials.28 The only absolute contraindication is hypersensitivity to the drug or any of its components.
It has not been evaluated in patients with LF. In patients with kidney failure, it is necessary to reduce the dose according to the glomerular filtration rate (GFR) (100 mg/12 h if GFR 50−70 mL/min/1.73 m2 and 100 mg/day if GFR 30−50 mL/min/1.73m2). Its use is not advised when the GFR is less than 30 mL/min/1.73m2.
Its effectiveness in children or adolescents younger than 17 years has not been established nor is there experience in patients older than 70 years. It crosses the placental barrier and is excreted in breast milk. Therefore, it should not be used during pregnancy or breastfeeding. Women of reproductive age must use contraceptive methods. Given that it affects sperm parameters, it is contraindicated in men who wish to conceive.15,28,48
Eliglustat is indicated for adult patients with GD1 who are PM, IM, or EM, but it cannot be used in those who are ultrarapid metabolizers (URM).49
It is contraindicated in cases of hypersensitivity to the drug or its components.49 It is likewise contraindicated in CYP2D6 IM and EM patients who take a strong or moderate inhibitor of said cytochrome, associated with another strong or moderate CYP3A4 inhibitor, or in CYP2D6 PM patients who take a strong CYP3A4 inhibitor.49,50 Due to its hepatic metabolism, it is contraindicated in EM patients with severe LF (Child-Pugh class C) or with mild/moderate LF (class A or B) associated with the administration of a strong/moderate CYP2D6 inhibitor.49
More information about the combination of eliglustat with other drugs and substances (grapefruit, etc.) is listed in the Tolerability section. Given that eliglustat uses lactose as an excipient, patients with a hereditary intolerance to galactose, total lactase deficiency, or glucose or galactose malabsorption should not take it.49
In patients with LF, apart from the aforementioned contraindications, eliglustat is not recommended in those who are CYP2D6 EM with moderate LF (Child-Pugh class B) or in patients who are IM or PM with any grade of LF. In patients who are EM with mild LF (class A) who take a mild CYP2D6 inhibitor or any CYP3A4 inhibitor, it is recommended to reduce the dose to a single 84-mg dose per day.49,51
A recent phase I study in a population with GD1 demonstrated that in patients who are EM with moderate LF, serum concentrations of eliglustat as well as its Cmax, AUC, and t1/2 are greater than in those with mild or no liver impairment.42 Pharmacokinetic models predict greater exposure to the drug in patients who are EM with mild LF and, most of all, those with moderate liver impairment compared to healthy controls. In addition, levels increase if CYP2D6 and CYP3A4 inhibitors are associated with it.42 The work concluded that mild liver disease in and of itself does not have a substantial effect on the pharmacokinetics of single-dose eliglustat in patients who are EM, but said effect was detected when the LF was moderate.42
In EM patients with kidney failure, eliglustat can be used without modifying the dose. The same phase I study in a population without GD142 concluded that exposure to eliglustat in EM subjects with severe kidney failure is similar to exposure in a healthy population. Its use is not recommended in patients who are IM or PM with any degree of renal impairment or for patients who are EM with terminal failure.49,51
In patients with cardiac impairment such as congestive heart failure, acute myocardial infarction, bradycardia, heart block, ventricular arrhythmia, or long QT syndrome, the use of eliglustat should be avoided as it can increase the QT interval when its plasma concentrations are substantially elevated (11 times the therapeutic dose).49–51 For this reason and due to drug interactions, the combination of eliglustat with antiarrhythmic drugs that lengthen the QT interval, such as class IA, class III,49,50 or class Ic drugs, must be avoided.51
In vitro data have demonstrated that eliglustat minimally inhibits the hERG potassium channel and the sodium Nav1.5 and calcium Cav1.2 channels.52 However, trials conducted in a healthy population (phase I) using supratherapeutic doses (800 mg) have not demonstrated significant electrocardiographic changes in the QT, QRS, or PR intervals.52 Given that a linear correlation has been demonstrated between the eliglustat concentration and QT changes, significant changes can be predicted if a strong CYP2D6 inhibitor and another similar CYP3A4 inhibitor are jointly administered (a combination which is contraindicated in the technical datasheet).52
In clinical practice, no cases of patients with clinically significant QT interval lengthening, changes in repolarization, torsade de pointes or sustained ventricular arrhythmia, second-degree AV block greater than type 1, or any other sign of cardiac toxicity have been detected either in the phase II and III trials (ENGAGE, ENCORE, and EDGE) or since the marketing of eliglustat.51,52 For all of the above, a cardiologic study is recommended prior to starting treatment with eliglustat.50
Experience with eliglustat in individuals older than 65 years is limited, but there do not appear to be differences compared to younger patients nor is it necessary to adjust the dose, although special attention must be paid to concomitant medication.49,50 Efficacy in children or those younger than 18 years has not been established. Therefore, its use is not recommended in these patients until the outcomes of specific clinical trials (ELIKIDS, NCT03485677) are obtained.49,50 Data on eliglustat in pregnant women are scarce and come from phase II and phase III (ENGAGE, ENCORE, and EDGE) and phase I (healthy population) CTs.53 It is considered a category C drug.51 Of the 202 women included in these four CTs, 18 women treated had 19 pregnancies with a median exposure to eliglustat of 38 days. Thirteen pregnancies reached term, resulting in 14 healthy children. There was only one miscarriage and one intrauterine fetal demise in the same patient; they were not related to eliglustat. Among the volunteers included in the phase I trial, one miscarriage occurred in a woman with a previous medical history of miscarriage.53 Although this miscarriage rate (5%) is lower than what is recorded in the general population (12%–24%), as a preventative measure, the use of eliglustat should be avoided in pregnancy and patients should switch to ERT.49,51
Data obtained from animal models show that eliglustat is secreted in breast milk. Therefore, the benefit to the mother must be weighed against that of the infant.49–51 It has been observed in animals that high doses of eliglustat can affect spermatogenesis in a reversible manner.49,50 However, data obtained from the four phase I and II CTs have demonstrated that pregnancy is possible in the female partners of men treated with eliglustat. Among the 393 patients included in these four CTs, 16 men who received treatment became fathers of 18 healthy children.53
Temporarily suspending eliglustat is recommended in patients who are going to undergo surgery.50
In summary, miglustat is a second-line treatment indicated for a small group of patients with GD1 in whom first-line treatment is not possible due to medical, access, or therapeutic compliance reasons.15,23,28,48 On the contrary, eliglustat is a first-line treatment similar to ERT which is applicable to all patients with GD1 who are not URM, provided that the patient’s concomitant medication or severe LF do not contraindicate it.49–51
Follow-upFollow-up on patients treated with eliglustat varies little compared to those who receive ERT.7,54,55 A cardiologic evaluation must be conducted prior to starting treatment, quantifying the phenotype by means of the Zimram,56 GauSSI-I,57 and GD-DS358 severity indexes as well as through the use of biomarkers, an MRI of the bone, and a bone density scan. All patients must undergo a routine comprehensive evaluation whose frequency depends on whether therapeutic objectives have been achieved, as follows:
- •
Until objectives are achieved:
- ◦
Every three months: hematological and biomarker evaluation.
- ◦
At 12 months: physical examination, SF-36 survey, CT scan or MRI of the liver and spleen, MRI of the skeleton, and a bone density scan.
- ◦
- •
Once objectives are reached:
- ◦
Annually: physical examination and SF-36 survey.
- ◦
Annually or biannually (individualized): hematological evaluation, biomarkers, CT scan or MRI of the liver and spleen, MRI of the skeleton, and a bone density scan.
- ◦
In addition, a comprehensive, immediate re-evaluation must be performed in the event a significant clinical complication occurs.
In regard to biomarkers, in phase II studies, all patients showed a very significant decrease with eliglustat starting in the first year and stabilization or slight decreases after the fourth year. Lyso-GL1 showed a 60% decrease in the first year and reached 80% after the second year. The decrease stabilized at 92% of the result obtained at diagnosis by the eighth year.31 What cannot be expected, neither with eliglustat nor with any other treatment, is to achieve normal figures for any biomarker and thus, this should not be a therapeutic objective.7,54
TolerabilityAs has been shown in these GD1 oral treatment guidelines, although two drugs approved for oral use are available for this disease (miglustat and eliglustat), both are very different in regard to their efficacy and tolerability. Therefore, as it is a first-line treatment, we will discuss specific tolerability issues of eliglustat.
Eliglustat and the CYP2D6 genotypeEliglustat is a drug that is metabolized hepatically by the cytochrome CYP2D6 and, to a lesser extent, the cytochrome CYP3A4. Table 2 shows some genetic variants of GYP2D6 with their corresponding metabolic activity.59
Therefore, before indicating treatment with eliglustat for a patient, we must know his or her CYP2D6 metabolic phenotype.49 This step is critical for starting and establishing treatment, given that based on said metabolic phenotype, we will know: (1) if the patient can receive treatment or not and (2) the appropriate dose for each patient. As previously stated in the Mechanisms of Action section, the treatment indication according to the phenotype and drug dose are shown in Table 3.59,60
The obligatory nature of determining the metabolic phenotype of a patient who is going to receive eliglustat, far from being a disadvantage, brings us closer to the use of pharmacogenetics as an aid in so-called personalized or precision medicine.60 This way, we know beforehand not only which patients will benefit from the drug and which will not, but also we can determine the appropriate dose for achieving the therapeutic objectives and how to modify it according to the administration of other drugs that share the same metabolic pathway.
Drug interactionsDrugs metabolized through the cytochromes CYP2D6 and CYP3A4 can act as simple substrates of the enzyme, either inducers or inhibitors of them. Therefore, there are drugs that can alter the metabolic capacity of said cytochromes, modifying the final concentration of eliglustat available after a given dose with a variable degree of interaction based on the patient’s metabolic phenotype.
In general, the use of eliglustat together with a strong CYP2D6 or CYP3A4 inhibitor would make the drug degrade more slowly, with an accumulation of the active drug and a decrease in its inactive metabolites. This may expose the patient to greater toxicity. On the other hand, the use of eliglustat with strong CYP3A4 inducers would make the drug degrade more than usual, with this effect translating into a decrease or absence of the desired therapeutic effect.60
By way of a summary, Table 4 allows us to establish the indications for use and the dose of eliglustat required when it is used concomitantly with drugs that share the same metabolic pathway.50,51,60,61
Dose of eliglustat according to the use of other possible concomitant drugs.
| Associated drug | Use/Recommended dose | ||
|---|---|---|---|
| Extensive metabolizer | Intermediate metabolizer | Poor metabolizer | |
| Strong CYP2D6 inhibitor (e.g., paroxetine, fluoxetine, quinidine, bupropion) | Not recommended | Not recommended | No data. Avoid |
| Moderate CYP2D6 inhibitor (e.g., duloxetine, terbinafine, moclobemide, mirabegron, cinacalcet, dronedarone) | 84 mg/24 h | 84 mg/24 h | No data. Avoid |
| Strong CYP3A4 inhibitor (e.g., ketoconazole, clarithromycin, itraconazole, cobicistat, indinavir, lopinavir, ritonavir, saquinavir, telaprevir, tipranavir, posaconazole, voriconazole, conivaptan, boceprevir) | 84 mg/24 h | Contraindicated | Contraindicated |
| Moderate CYP3A4 inhibitor (e.g., fluconazole, erythromycin, ciprofloxacin, diltiazem, verapamil, aprepitant, atazanavir, darunavir, fosamprenavir, imatinib, cimetidine) | 84 mg/24 h | 84 mg/24 h | Not recommended |
| CYP2D6 + CYP3A4 inhibitor (M o F) | Contraindicated | Contraindicated | Contraindicated |
| CYP3A4 inducer (e.g., rifampicin, rifampin, rifabutin, phenobarbital, carbamazepine, phenytoin, dexamethasone, St. John's wort) | Not recommended | Not recommended | Not recommended |
Likewise, the use of eliglustat may condition the concentration of other drugs prescribed for the same patient and which use either the CYP2D6 or P-glycoprotein pathway. Therefore, given the possible need to use some of these drugs in a patient with GD1 who takes eliglustat, a potential adjustment to his or her dose should be considered.
In Table 5, we present some examples of drugs that use the CYP2D6 or P-glycoprotein pathway during their metabolism.
Drugs metabolized through CYP2D6 and the P-GP pathway.
| Drugs metabolized as substrates CYP2D6 pathway | |
|---|---|
| Amphetamines | Amphetamine, dexfenfluramine, methoxyamphetamine |
| Antiarrhythmics | Lidocaine, encainide, flecainide, mexiletine, propafenone, quinidine |
| Antidepressants | Amitriptyline, clomipramine, imipramine, desipramine, nortriptyline, paroxetine, atomoxetine, venlafaxine |
| Antipsychotics | Chlorpromazine, clozapine, haloperidol, risperidone, thioridazine, zuclopenthixol, quetiapine, aripiprazole, ziprasidone |
| β-blockers | Alprenolol, carvedilol, labetalol, metoprolol, propranolol, timolol |
| Opioids | Codeine, ethylmorphine, hydrocodone, tramadol, dextromethorphan |
| Others | Chlorpropamide, flunarizine, tamoxifen, ondansetron, tropisetron, chlorpheniramine, loratadine |
| Drugs which pass through the P-GP | |
| Digoxin, dabigatran, colchicine, tacrolimus, quinidine, dexamethasone | |
P-GP, P-glycoprotein.
Other online resources for drugs that have not been described can be consulted, including: www.uptodate.com, www.drugs.com, and https://reference.medscape.com/drug-interactionchecker.
Adverse effectsThe most frequently documented side effects related to treatment were dyspepsia, headache, abdominal pain, and instability. They have been reported in 5 %–6 % of all patients studied. Most of these events were mild and were documented only occasionally in most patients. In addition, as the time a patient is in treatment with eliglustat increases, fewer adverse effects are reported.30,32–34
There are no differences in the onset of side effects in the different metabolic phenotypes (extensive, intermediate, or poor metabolizers), which shows the efficacy of the prior determination of the CYP2D6 genotype in patients who are going to receive the drug.
In regard to adverse effects associated with eliglustat are compared to those related to miglustat, no class effect is observed in substrate reduction therapy. The toxicity documented with miglustat (diarrhea (80%), weight loss (65%), and tremor (30%)) is hardly present in those taking eliglustat (14%, 0.8%, and 0.8%, respectively).
DiscussionThis article presents this working group’s recommendations on oral treatment of GD1 in adults. It should always be taken into account that each patient and their specific circumstances must be evaluated individually. Though the main limitation of this work is the scarce amount of evidence found—secondary to the low prevalence of GD1—it is useful because it is the first set of guidelines which compares and establishes different recommendations for the use of various oral drugs in GD1. Our intention is to regularly evaluate the evidence published in this field in case modifications to this document are necessary. If new scientific contributions which come into conflict with what is described in these guidelines are detected, a new edition will be created.
These recommendations will have a validity of two years since first publication and an update by means of a systematic literature review and by external reviewers is planned in November 2023. Those responsible for conducting the aforementioned review will be authors of this manuscript.
Given the characteristics of this consensus document, its applicability or utility cannot be evaluated.
ConclusionsThis document can be summarized in the following points:
- 1
The criteria for starting treatment in patients with GD1 must be evaluated individually and must follow the internationally recommended short- and long-term therapeutic objectives.
- 2
Miglustat and eliglustat are both GCst inhibitors, but have different mechanisms of action and pharmacological properties, which explains the differences in their effectiveness. This makes it obligatory to use them according to the indications on their technical datasheets and they must never be considered equivalent.
- 3
It is recommended to evaluate each drug’s indications before beginning treatment given that miglustat is indicated for patients with non-severe GD1 who cannot receive another first-line treatment whereas eliglustat is indicated for patients with GD1 of any degree of severity as a first-line treatment and without the need for previous stabilization with ERT.
- 4
It is not necessary to administer ERT prior to starting eliglustat. It can be started in patients regardless of GD1 severity.
- 5
Eliglustat is a drug that is metabolized hepatically by the cytochrome CYP2D6 and, to a lesser extent, the cytochrome CYP3A4. Therefore, before indicating treatment with eliglustat, we must determine the patient’s CYP2D6 metabolic phenotype. This step is critical for the start and dosing of treatment.
- 6
The use of eliglustat should be avoided during pregnancy; only ERT should be used.
- 7
Before beginning treatment with eliglustat, a cardiologic and bone evaluation should be conducted, quantifying the phenotype through severity indexes and the use of biomarkers. All patients must undergo a routine comprehensive evaluation whose frequency depends on whether therapeutic objectives have been reached.
- 8
The normalization of biomarkers must not be a therapeutic objective.
- 9
The association of eliglustat with drugs metabolized through the cytochromes CYP2D6 and CYP3A4—or those which use the P-glycoprotein pathway—must be done taking into account dose adjustments based on the eliglustat metabolizer phenotype and the degree of inhibition/induction of the associated drug.
- 10
Eliglustat has been demonstrated to improve the quality of life of patients with GD1.
The origin of this initiative for creating a protocol arose from a group of physicians who treat patients with GD1. The motivation for creating these recommendations comes from the need for unifying criteria for action and minimizing clinical variability in order to increase clinical safety; there have been no other influences on its content. Throughout the creation of this document, all authors have maintained editorial independence and independence of opinion. They received logistical support from the company Anima Consulting, S.L., which was hired by Sanofi España. Sanofi España had no direct relationship with the authors. None of the authors received direct funding from the industry.
Conflicts of interestThe authors of this text declare the following conflicts of interest:
Miguel Ángel Torralba-Cabeza has received research funding and participated in conferences funded by Genzyme-Sanofi® and Shire®.
Marta Morado-Arias has received compensation from Genzyme-Sanofi®, Agios®, Alexion®, Novo Nordisk®, and Novartis® for her participation as an expert on Advisory Boards and as a speaker at conferences.
Agustín Pijierro-Amador has received compensation for his participation in conferences funded by Genzyme-Sanofi® and Takeda-Shire®, Alexion® and Alnylan®.
María Cristina Fernández-Canal has received compensation for her participation in conferences funded by Genzyme-Sanofi®.
Jesús Villarrubia-Espinosa has received research funding and participated in conferences funded by Genzyme-Sanofi® and Takeda-Shire®.
Please cite this article as: Torralba-Cabeza MÁ, Morado-Arias M, Pijierro-Amador A, Fernández-Canal MC, Villarrubia-Espinosa J, Recomendaciones para el tratamiento oral de pacientes adultos con enfermedad de Gaucher tipo 1, Revista Clínica Española. 2022;222:529–542.