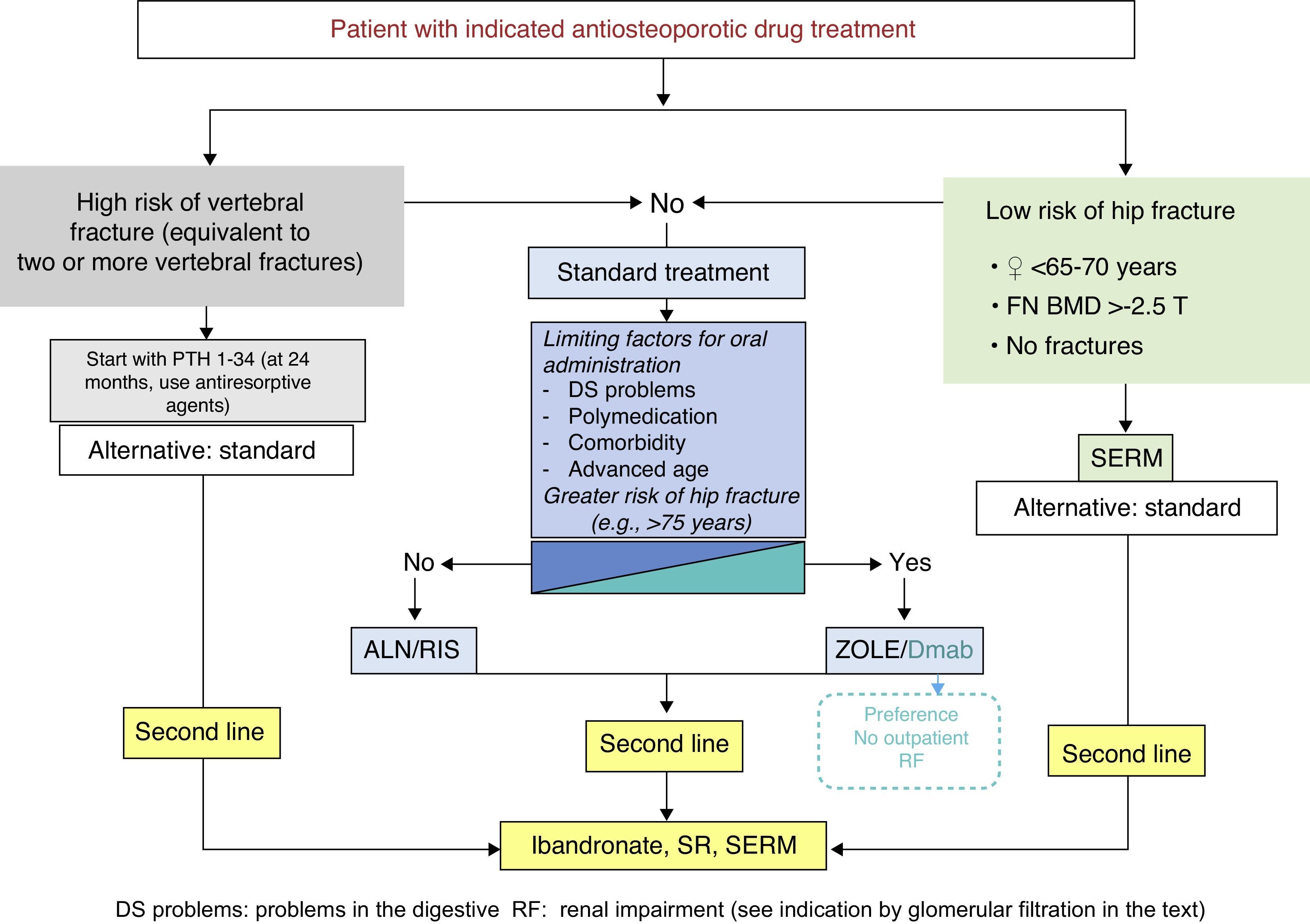
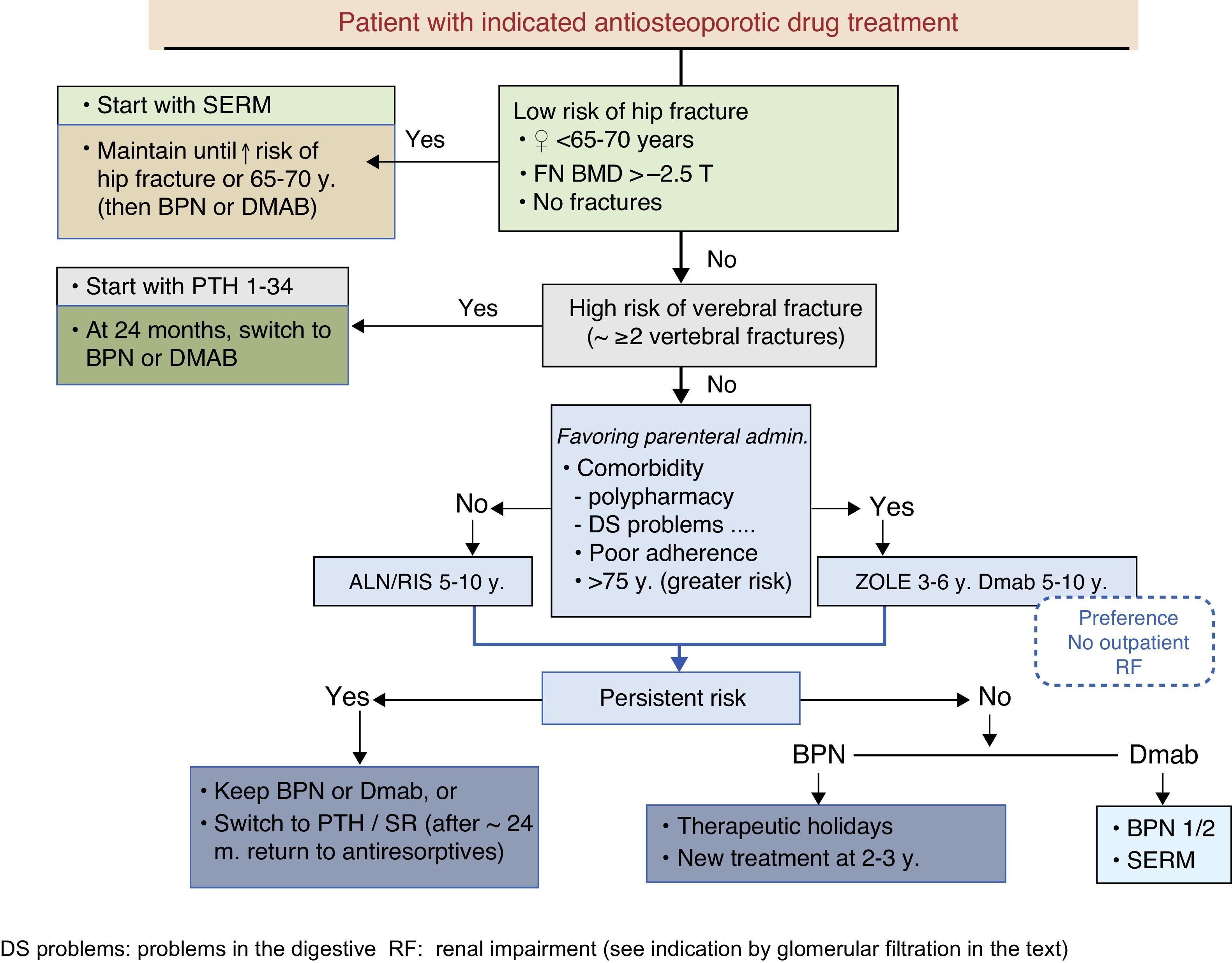
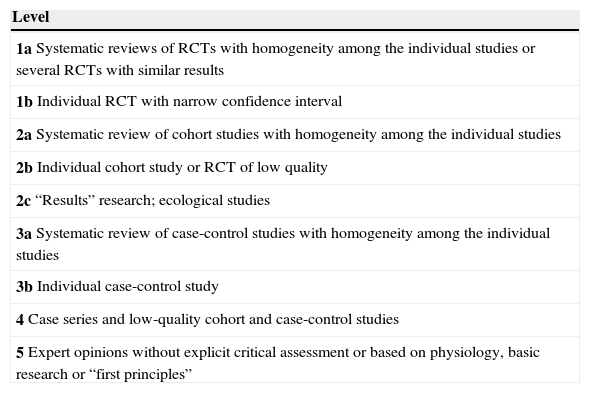
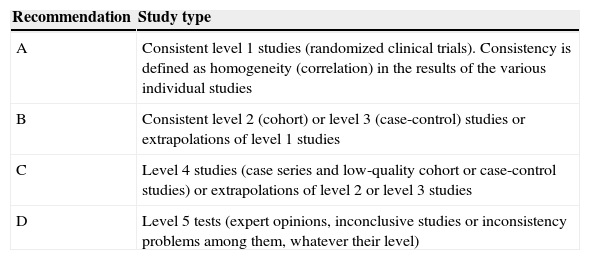
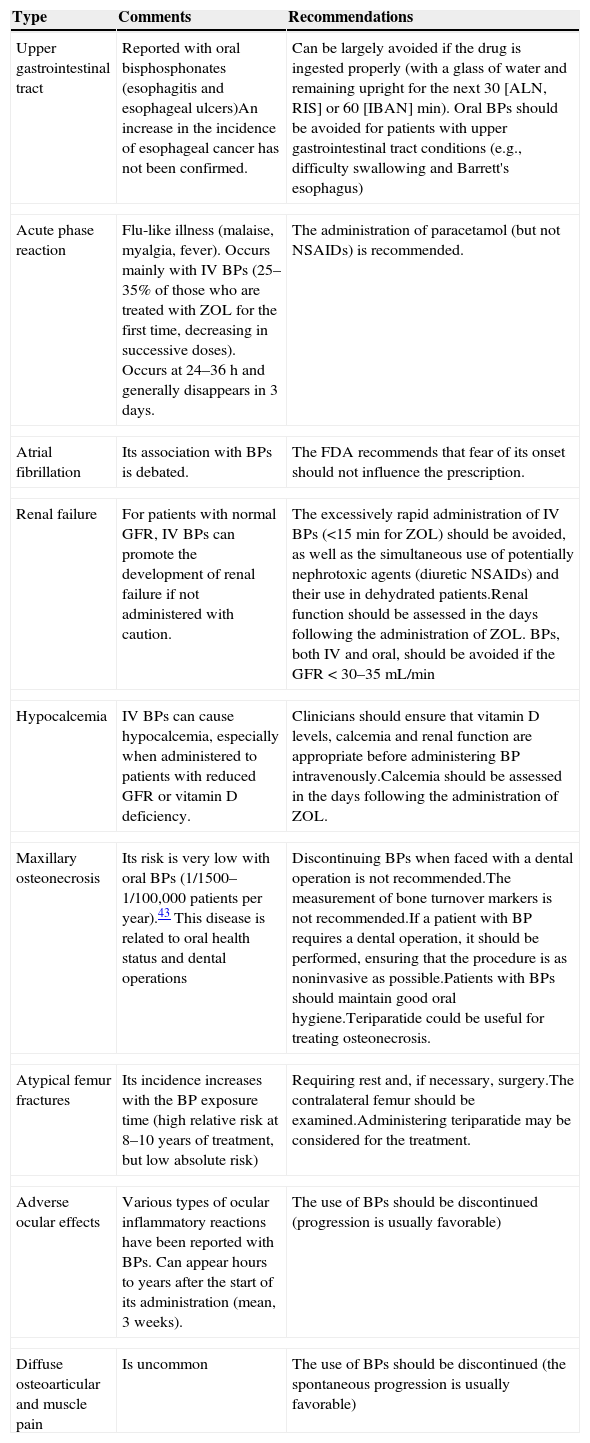
These guidelines update issues covered in previous versions and introduce new ones that have arisen in recent years. The former refer mainly to the therapeutic developments that have been made during this time (zoledronate, denosumab, bazedoxifene), which have led to a change in the drug selection algorithm. The latter deal with therapeutic management, the description of new adverse effects (which have led to changes in therapeutic behavior patterns, as is the case with atypical fracture of the femur), treatment duration (with consideration for the so-called “therapeutic holidays”), the so-called sequential treatment and changes in treatment imposed by certain circumstances. A new algorithm has been introduced for sequential treatment. Attention has also been paid to vertebroplasty and kyphoplasty.
La presente guía actualiza aspectos tratados en versiones anteriores, e introduce otros nuevos surgidos en los últimos años. Los primeros se refieren fundamentalmente a las novedades terapéuticas aparecidas en este tiempo (zoledronato, denosumab, bazedoxifeno), que han conducido a una modificación del algoritmo de elección del fármaco. Los segundos tienen que ver con el control terapéutico, la descripción de nuevos efectos secundarios (que han condicionado cambios en los patrones de conducta terapéutica, como es el caso de la fractura atípica de fémur), la duración del tratamiento (con la consideración de las denominadas «vacaciones terapéuticas»), los cambios de tratamiento que pueden imponer unas u otras circunstancias, y el denominado tratamiento secuencial. En relación con este último se ha introducido un nuevo algoritmo. También se han incluido consideraciones respecto a la vertebroplastia y la cifoplastia.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<