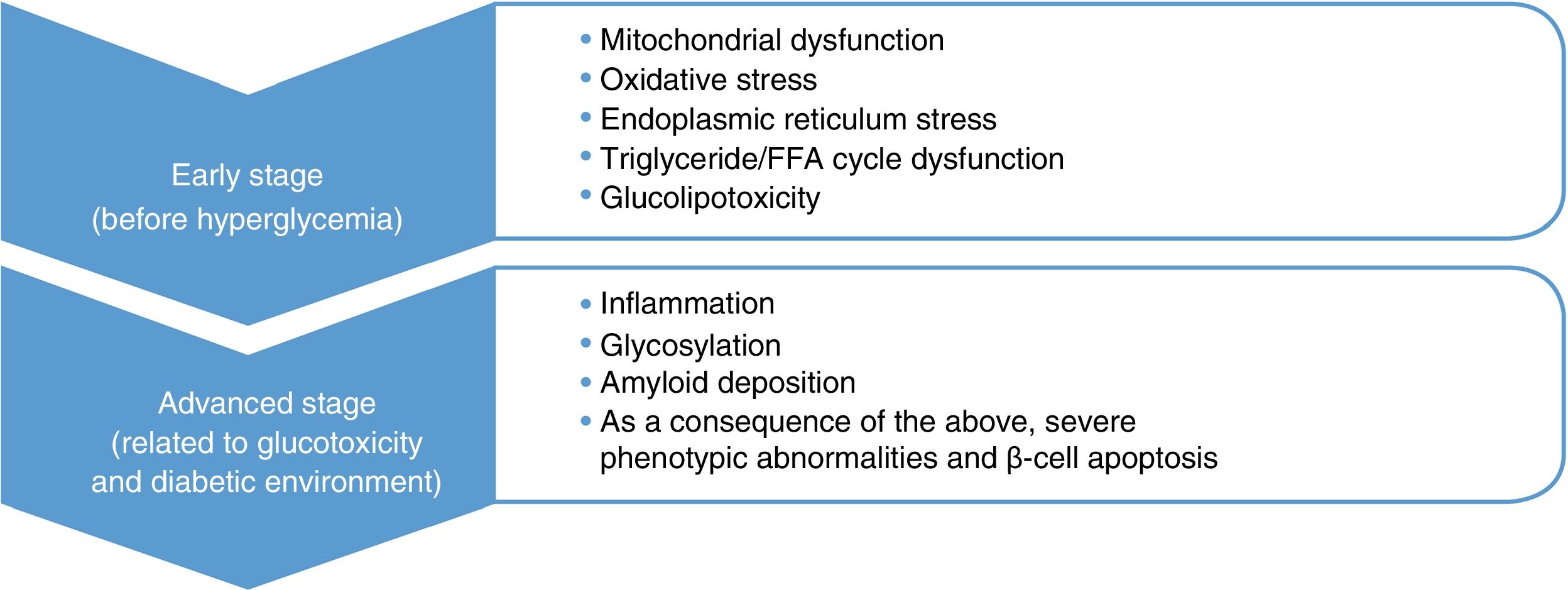
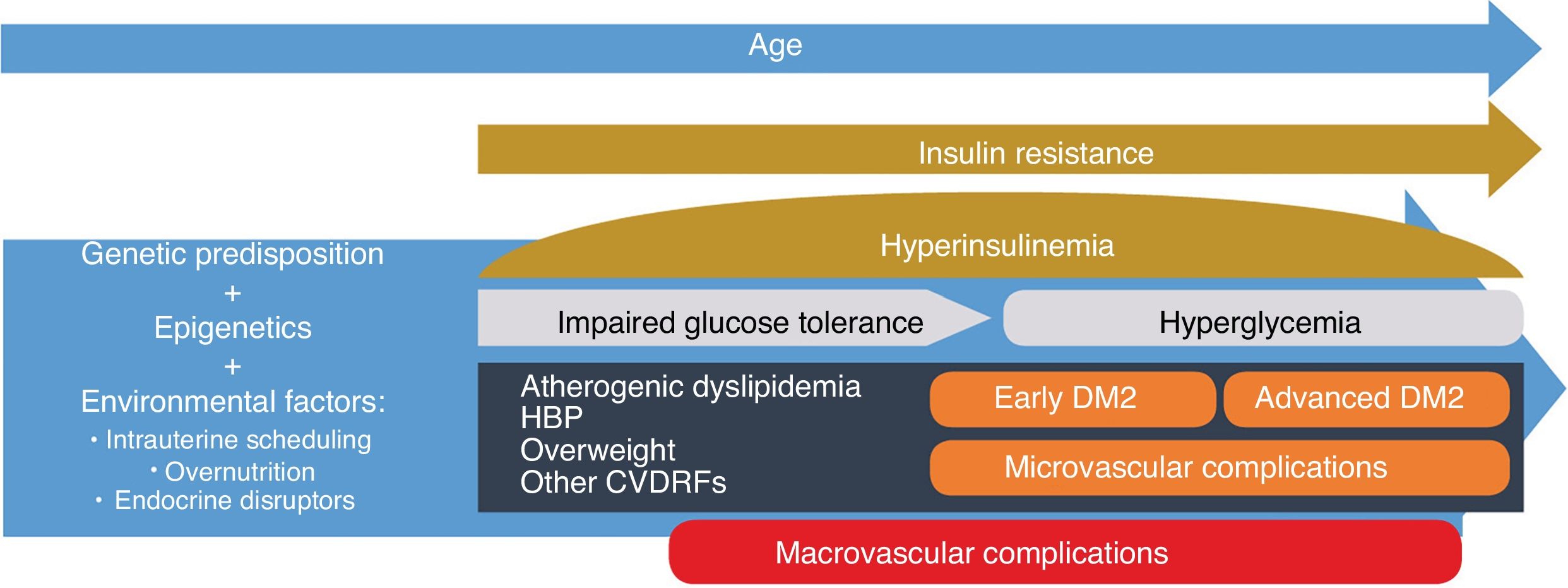
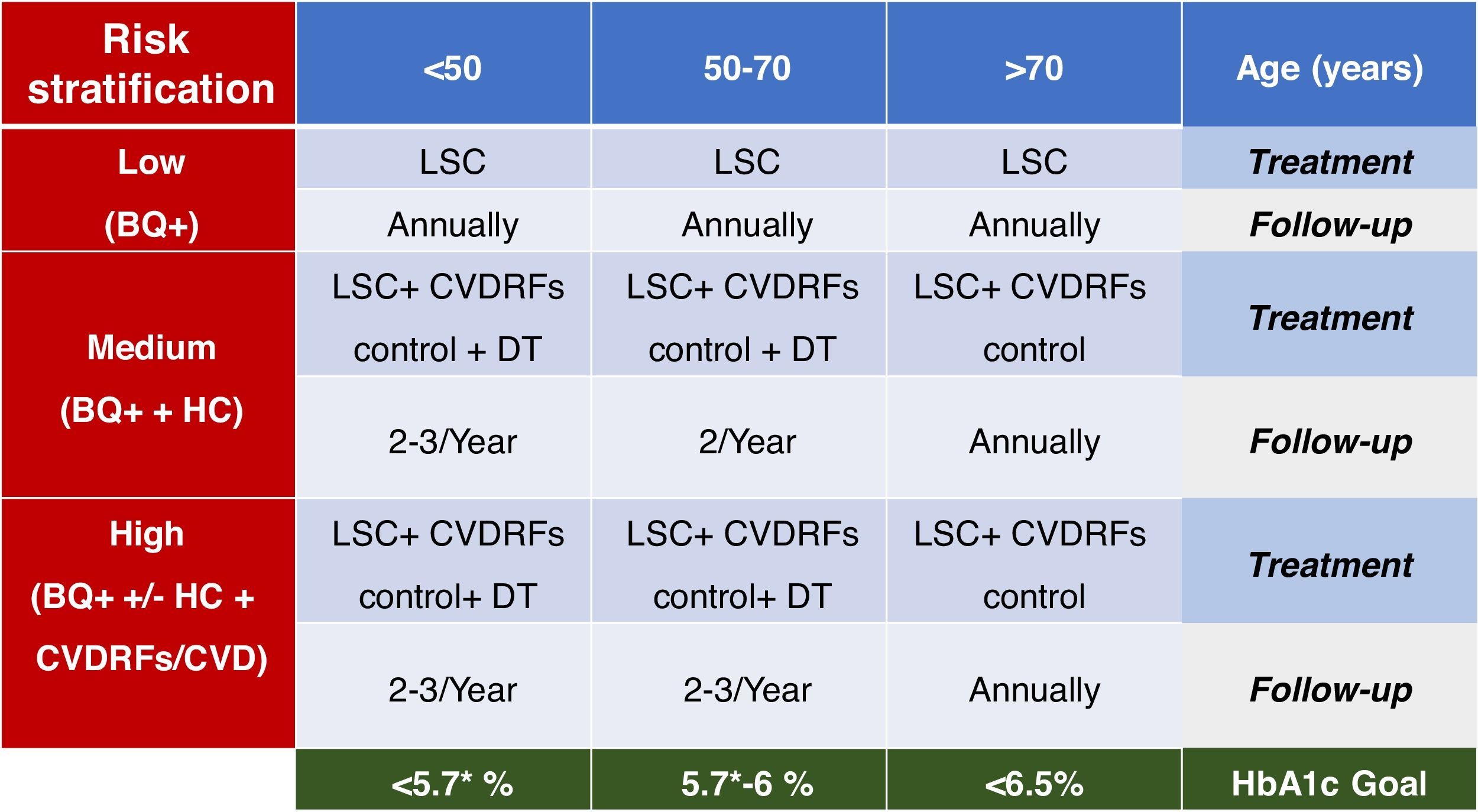
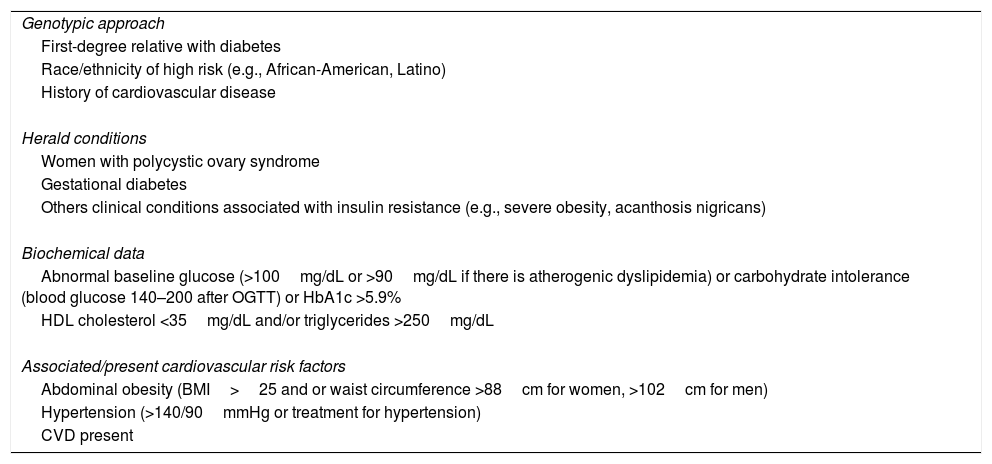
Type 2 diabetes mellitus (DM2) is a progressive disease whose pathophysiological changes occur several years before its detection. An approach based on the pathophysiological development of DM2 and its complications emphasises the importance of early and intensive intervention, not only to prevent beta-cell dysfunction but also to act on the potential associated cardiovascular risk factors before reaching the blood glucose thresholds currently set for diagnosing DM2. In the field of recently diagnosed DM2, the VERIFY study has shown that early treatment combined with metformin-vildagliptin provides relevant improvements in long-term glycaemic control and can positively affect the disease's progression.
La diabetes mellitus tipo 2 (DM2) es una enfermedad progresiva cuyos cambios fisiopatológicos se producen varios años antes de su detección. Un abordaje basado en el desarrollo fisiopatológico de la DM2 y sus complicaciones enfatiza la importancia de una intervención temprana e intensiva, no solo para prevenir la disfunción de las células β, sino también para actuar sobre los posibles factores de riesgo cardiovascular asociados antes de alcanzar los umbrales glucémicos fijados actualmente para el diagnóstico de la DM2. En el terreno de la DM2 de reciente diagnóstico, el estudio VERIFY ha mostrado que el tratamiento precoz combinado con metformina-vildagliptina proporciona mejoras relevantes en el control glucémico a largo plazo y puede influir positivamente en la evolución de la enfermedad.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<