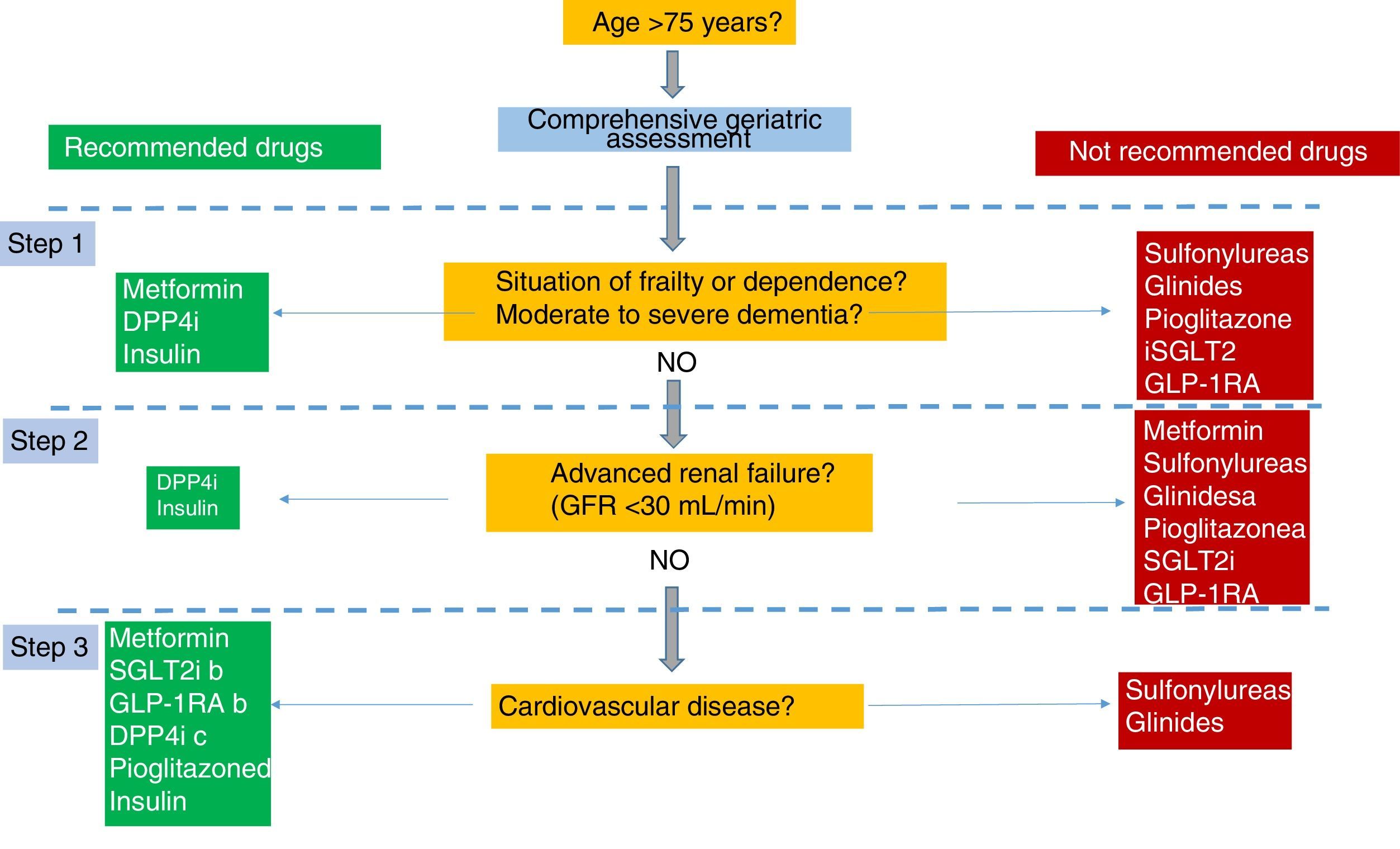
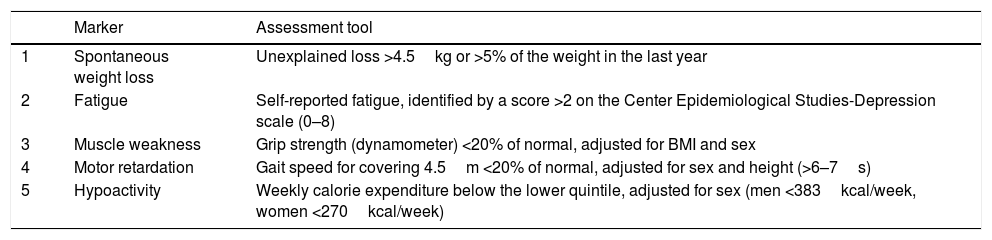
The prevalence of type 2 diabetes mellitus (DM2) increases markedly with age. Antidiabetic treatment and the objectives of glycaemic control in elderly patients with DM2 should be individualized according to their biopsychosocial characteristics. In elderly patients for whom the benefits of intensive antidiabetic treatment are limited, the basic objectives should be to improve the quality of life, preserve functionality and avoid adverse effects, especially hypoglycaemia. Treatment of DM2 in the elderly was the subject of a consensus document published in 2012 and endorsed by several Spanish scientific societies. Since then, new therapeutic groups and evidence have emerged that warrant an update to this consensus document. The present document focuses on the therapeutic aspects of DM2 in elderly patients, understood as being older than 75 years or frail.
La prevalencia de la diabetes mellitus tipo 2 (DM2) se incrementa marcadamente con la edad. El tratamiento antidiabético y los objetivos de control glucémico en el anciano con DM2 deben individualizarse en función de sus características biopsicosociales. En los pacientes de edad avanzada, en los que los beneficios de un tratamiento antidiabético intensivo son limitados, los objetivos básicos deben ser mejorar la calidad de vida, preservar la funcionalidad y evitar los efectos adversos, muy especialmente las hipoglucemias. El tratamiento de la DM2 en el anciano fue objeto de un consenso, publicado en 2012 y avalado por varias sociedades científicas españolas. Desde entonces, han aparecido nuevos grupos terapéuticos y evidencias que hacen recomendable su actualización. El presente documento se centrará en los aspectos terapéuticos de la DM2 en el paciente anciano, entendiendo como tal el tener una edad mayor de 75 años o presentar fragilidad.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<