Emerging evidence suggests that frailty may be a significant predictor of poor outcomes in older individuals hospitalized due to COVID-19. This study aims to determine the prognostic value of frailty on intrahospital patient survival.
MethodsThis observational, multicenter, nationwide study included patients aged 70 years and older who were hospitalized due to COVID-19 in Spain between March 1 and December 31, 2020. Patient data were obtained from the SEMI-COVID-19 Registry of the Spanish Society of Internal Medicine. Frailty was assessed using the Clinical Frailty Scale. The primary outcome was hospital survival. Cox proportional hazards models were used to assess predictors of survival.
ResultsA total of 1,878 participants (52% men and 48% women) were included, with 1,351 (71.9%) survivors and 527 (28.1%) non-survivors. The non-survivor group had higher mean age (83.5 vs. 81 years), comorbidities (6.3 vs. 5.3 points on the Charlson index), degree of dependency (26.8% vs. 12.4% severely dependent patients), and frailty (34.5% vs. 14.7% severely frail patients) compared to survivors. However, there were no differences in terms of sex. Our results demonstrate that a moderate-severe degree of frailty is the primary factor independently associated with shorter survival [HR 2.344 (1.437−3.823; p<0.001) for CFS 5−6 and 3.694 (2.155−6.330; p<0.001) for CFS 7−9].
ConclusionFrailty is the main predictor of adverse outcomes in older patients with COVID-19. The utilization of tools such as the Clinical Frailty Scale is crucial for early detection in this population.
La evidencia reciente sugiere que la fragilidad puede ser un importante predictor de resultados adversos en personas mayores hospitalizadas por COVID-19. El objetivo de este estudio es determinar el valor pronóstico de la fragilidad en la supervivencia intrahospitalaria de estos pacientes.
MétodosEstudio observacional, multicéntrico y de ámbito nacional de pacientes ≥70 años hospitalizados a consecuencia de la COVID-19 en España desde el 1 de marzo hasta el 31 de diciembre de 2020. Los datos de los pacientes se obtuvieron del Registro SEMI-COVID-19 de la Sociedad Española de Medicina Interna. Se utilizó la Escala de Fragilidad Clínica para evaluar la fragilidad. El resultado primario fue la supervivencia hospitalaria. Se realizó un modelo de riesgos proporcionales de Cox para evaluar los predictores de supervivencia.
ResultadosSe incluyeron 1.878 participantes (52% hombres y 48% mujeres). 1.351 (71,9%) supervivientes y 527 (28,1%) no supervivientes. El grupo de no supervivientes presentaba en comparación con los supervivientes una media de edad superior (83,5 frente a 81 años), más comorbilidades (6,3 frente a 5,3 puntos en el índice de Charlson), mayor grado de dependencia (26,8% frente a 12,4% de pacientes con dependencia severa) y de fragilidad (34,5% frente a 14,7% de pacientes con fragilidad severa), sin embargo, no hubo diferencias en cuanto al sexo. Nuestros resultados muestran que un grado de fragilidad moderado-grave es el principal factor asociado de forma independiente con una menor supervivencia [HR 2,344 (1,437−3,823; p<0,001) para SFC 5−6 y 3,694 (2155−6,330; p<0,001) para SFC 7−9.].
ConclusionesLa fragilidad es el principal predictor de resultados adversos en pacientes mayores con COVID-19. El uso de herramientas como la CFS es fundamental para la detección precoz de fragilidad en esta población.
The COVID-19 pandemic has had an immense impact on the elderly, who are among the most vulnerable and affected groups. This population experiences a higher proportion of severe cases and complications during infection 1 and exhibits intrinsic and extrinsic factors that contribute to increased clinical fragility and susceptibility to infectious processes, such as weakened immune systems or immunosenescence,2 heightened comorbidity,3 malnutrition,4 and a higher rate of institutionalization.
Since the onset of the COVID-19 pandemic, advanced age has been identified as one of the strongest risk factors for poor outcomes, complications, and mortality.5–7 Age is an easily measurable prognostic marker; however, its prognostic utility by itself is limited.8 In this regard, emerging evidence suggests that frailty may be a significant predictor of poor outcomes in older people hospitalized due to COVID-19.9,10 Frailty has also been used for clinical decision-making during this pandemic,11 but further clinical research is still needed to determine the usefulness of frailty screening in predicting adverse disease.
Frailty is defined as "a medical syndrome with multiple causes and contributors characterized by diminished strength, endurance, and reduced physiological function, which increases an individual's vulnerability to developing increased dependency and/or death".12 The likelihood of frailty increases with age, estimated to affect around 40% of older patients.13 Fried's frailty criteria are widely used for diagnosing frailty.14 According to these criteria, a diagnosis of frailty is established if the patient meets three of the following criteria: unintentional weight loss, exhaustion, muscle weakness, motor slowness, and low activity. Over the years, multiple instruments have been developed to assess frailty, including rapid detection scales that are more feasible in clinical practice and require only a few minutes of application, such as the Clinical Frailty Scale (CFS)15 and the FRAIL scale.16
The relationship between the degree of pre-infection clinical frailty and the progression of COVID-19 has been the subject of several studies to date. However, most studies assessing the prognostic capacity of clinical frailty have either been conducted in the general population or, if they do evaluate prognostic factors in older patients, have not included the degree of frailty. These studies have examined the relationship between frailty and mortality, hospital infection rates, intensive care admission rates, and disease phenotypes.10,17,18
Other factors, such as advanced age, male sex, severe functional dependence, and comorbidities like hypertension, diabetes mellitus, and obesity, as well as analytical parameters (C-reactive protein, lymphopenia, neutrophilia, etc.), clinical parameters at admission (hypoxia, high SOFA score, temperature, etc.), and the presence of radiological abnormalities, have also been identified as the main risk factors for poor outcomes in older people with COVID-19 infection.19–21
Systematic frailty assessment in older patients with COVID-19 infection allows for the early identification of frail elderly patients. This enables better care for those at higher risk of severe disease and facilitates improved resource allocation. Therefore, the objectives of this study in older patients hospitalized due to COVID-19 infection are as follows: a) to determine the prognostic value of frailty on intrahospital patient survival compared to other previously identified predictors of poor prognosis, and b) to emphasize the importance of early detection of frailty in this population.
Materials and methodsStudy design and recruited populationThis was an observational, multicenter, nationwide study of patients aged ≥70 years old who were hospitalized due to COVID-19 in Spain from March 1 to December 31, 2020. Patient data was obtained from the Spanish Society of Internal Medicine's SEMI-COVID-19 Registry, which includes 150 Spanish hospitals. The registry encompasses all consecutive patients aged ≥18 years old admitted to hospitals with confirmed COVID-19 through microbiological testing using reverse transcription polymerase chain reaction (RT-PCR) on nasopharyngeal swab samples, sputum specimens, or bronchoalveolar lavage. For this study, we focused on the subpopulation of patients aged ≥70 years old.
Definition of variablesThe SEMI-COVID-19 Registry retrospectively collects data from the initial admission of patients aged ≥18 years with confirmed COVID-19. The data include sociodemographic information, previous medical history, routine treatments, clinical presentation, clinical condition, laboratory test results, radiological findings, clinical management, in-hospital complications, length of hospital stay, early readmissions, referral to long-term care or skilled nursing facilities, and in-hospital deaths. More detailed information about the justification, objectives, methodology, and preliminary results of the SEMI-COVID-19 Registry has been published in this journal (Vol. 220. No. 8.).22 Clinicians collected the data retrospectively using an online electronic data capture system.
To assess preadmission functional status, we used the Barthel Index. A score of 100−91 indicates independence or slight dependency, 90−61 indicates moderate dependence, and ≤60 indicates severe dependency.23 The comorbidity burden was assessed using the age-adjusted Charlson Comorbidity Index (CCI).24 The diagnosis of dementia was based on DSM-5 criteria.25 Atherosclerotic cardiovascular disease encompassed a history of ischemic cardiopathy (myocardial infarction, acute coronary syndrome, angina, or coronary revascularization), cerebrovascular disease (stroke, transient ischemic attack), or peripheral arterial disease (intermittent claudication, revascularization, lower limb amputation, or abdominal aortic aneurysm). Nonatherosclerotic cardiovascular disease included atrial fibrillation and heart failure. Obesity was defined as a body mass index ≥30kg/m2. Hypertension, diabetes mellitus, and dyslipidemia were considered present if there was a prior clinical diagnosis or if the patients had been receiving pharmacological treatment for these conditions. Chronic pulmonary disease was defined as a diagnosis of chronic obstructive pulmonary disease and/or asthma. Malignancy included solid tumors and/or hematologic neoplasia (excluding nonmelanoma skin cancer). Moderate-to-severe renal disease was defined as an estimated glomerular filtration rate <45mL/min/1.73m2 according to the CKD-EPI equation.26
Preadmission comorbidity data were collected from each patient's electronic medical record at each hospital. Laboratory data (blood gases, metabolic panel, complete blood count, coagulation) and diagnostic imaging tests were collected at admission.
The variables for analysis were selected based on recent studies on COVID-19 that identified them as indicators of poor prognosis.19–21 These variables included age, male sex, level of severe dependence (Barthel <60), clinical diagnosis of coronary heart disease, diabetes, and hypertension; smoking (previous or current), oxygen saturation <90%, temperature ≥37.8°C at admission, and blood biomarkers (lactate dehydrogenase (LDH) ≥500 U/L, C-reactive protein (CRP) ≥80mg/L, neutrophil count ≥7.5×103/μL, and lymphocyte count <0.800×103/μL) as well as bilateral pulmonary infiltrates on chest X-ray.
Frailty was assessed using the Clinical Frailty Scale (CFS).15 The assessment was based on the patient's condition two weeks before hospital admission. The CFS is an ordinal hierarchical scale that ranks frailty from 1 to 9, with a score of 1 indicating very fit, 2 indicating well, 3 indicating managing well, 4 indicating vulnerable, 5 indicating mildly frail, 6 indicating moderately frail, 7 indicating severely frail, 8 indicating very severely frail, and 9 indicating terminally ill. Due to the inadequate number of events for each score, the scores were grouped as follows for analysis: 1–2 (fit), 3–4 (becoming vulnerable, but not frail), 5–6 (initial signs of frailty but with some degree of independence), and 7–9 (severe or very severe frailty). These groupings were selected to align with the clinical descriptions outlined in the CFS and were considered reasonable severity groupings for frailty.
The primary outcome of the study was intrahospital survival, defined as the time from hospital admission due to COVID-19 to in-hospital mortality. For patients diagnosed with COVID-19 while already being hospitalized (hospital-acquired or nosocomial infection), the date of diagnosis was used instead of the date of admission.
Statistical analysisQualitative variables were presented as absolute and relative frequencies and compared using the chi-square test or Fisher's exact test, as appropriate. Quantitative variables were expressed as mean and standard deviation and compared using Student's t-test for independent groups.
To assess the prognostic value of frailty on patient survival, a multivariate Cox proportional hazards analysis was conducted. This analysis included variables that showed a significant association in the univariate analysis, as well as other variables of recognized prognostic value and potentially confounding factors reported in the literature, particularly those found in a previous article based on this COVID-19 patient registry.19 Kaplan–Meier curves were generated to visually represent patient survival according to frailty categories. A significance level of 0.05 (95% confidence level) was assumed. The data were stored and analyzed using the SPSS statistical package, version 25, for Windows.
Ethical aspectsInformed consent was obtained from all patients. In cases where biosafety concerns or patient discharge had occurred, verbal informed consent was requested and documented in the medical records. Data confidentiality and patient anonymity were strictly maintained in accordance with Spanish regulations governing observational studies. Patient identifiable information was removed before analyzing the database, ensuring that individual patients cannot be identified either in this article or in the database.
ResultsIn the SEMI-COVID-19 Registry, a total of 1,920 patients aged ≥70 years who had been hospitalized due to COVID-19 infection between March 1 and December 31, 2020 were identified, and their degree of frailty was assessed. Forty-two participants were excluded due to incomplete registration of minimum clinical characteristics. Ultimately, the study included 1,878 participants of both sexes, with 52% men and 48% women. Among the included patients, 1,351 (71.9%) were discharged alive from the hospital, while 527 (28.1%) died during their hospital stay. There were no significant differences in the survival rates between males and females (Table 1).
Clinical and demographical characteristics in patients ≥70 years hospitalised due to COVID-19.
| Variables | All patients (n=1878) | Dead (n=527) | Alive (n=1351) | p |
|---|---|---|---|---|
| Age, years (mean±standard deviation) | 81.7±6.9 | 83.5±7.0 | 81.0±6.8 | <0.001 |
| Sex n (%) | 0.012 | |||
| Female | 901 (48.0) | 228 (43.3) | 673 (49.8) | |
| Male | 977 (52.0) | 299 (56.7) | 678 (50.2) | |
| BMI (mean±standard deviation) | 28,8±4.9 | 29.0±5.4 | 28.7±4.7 | 0.570 |
| Smoking status n (%) | 581 (31.3) | 185 (35.7) | 396 (29.6) | 0.010 |
| Comorbidities n (%) | ||||
| Arterial hypertension | 1412 (75.2) | 412 (78.2) | 1000 (74) | 0.061 |
| Dyslipidaemia | 974 (51.9) | 299 (56.7) | 288 (43.3) | 0.008 |
| Diabetes without target organ damage | 415 (22.1) | 108 (20.5) | 307 (22.7) | 0.295 |
| Diabetes with target organ damage | 187 (10.0) | 75 (14.2) | 112 (8.3) | <0.001 |
| Atrial fibrillation | 395 (21.0) | 145 (27.5) | 250 (18.5) | <0.001 |
| Dementia | 390 (20.8) | 165 (31,3) | 225 (16.7) | <0.001 |
| Degenerative neurological disease | 322 (17.2) | 127 (24.2) | 195 (14.4) | <0.001 |
| Heart failure | 221 (11.8) | 100 (19.0) | 121 (9.0) | <0.001 |
| COPD | 195 (10.4) | 65 (12.4) | 130 (9.6) | 0.082 |
| Moderate-severe Chronic renal failure | 184 (9.8) | 86 (16.3) | 98 (7.3) | <0.001 |
| Acute myocardial infarction | 173 (9.2) | 78 (14.8) | 95 (7.0) | <0.001 |
| Peripheral vascular disease | 114 (6.1) | 41 (7.8) | 73 (5.4) | 0.054 |
| Asma | 113 (6.0) | 29 (5.5) | 84 (6.2) | 0.558 |
| Obstructive sleep apnea syndrome | 104 (5.6) | 32 (6.1) | 72 (5.4) | 0.529 |
| Cerebrovascular disease | 90 (4.8) | 40 (7.6) | 50 (3.7) | <0.001 |
| Neoplasia with metastasis | 38 (2.0) | 16 (3.0) | 22 (1.6) | 0.052 |
| Charlson Index (mean±standard deviation) | 5.6±2.0 | 6.3±2.2 | 5.2±1.9 | <0.001 |
| Level of dependency n (%) | <0.001 | |||
| Mild | 1171 (62.5) | 231 (43.9) | 940 (69.7) | |
| Moderate | 395 (21.1) | 154 (21.1) | 241 (17.9) | |
| Severe | 114 (16.4) | 141 (26.8) | 167 (12.4) | |
| CFS n (%) | <0.001 | |||
| 1−2: very fit/fit | 174 (9.3) | 20 (3.8) | 154 (11.4) | |
| 3−4: managing well/vulnerable | 795 (42.3) | 145 (27.5) | 650 (48.1) | |
| 5−6: mildly frail/moderately frail | 529 (28.2) | 180 (34.2) | 349 (25.8) | |
| 7−9: severely frail/very severely frail/terminally ill | 380 (20.2) | 182 (34.5) | 198 (14.7) |
BMI, body mass Index; COPD, chronic obstructive pulmonary disease; CFS, clinical frailty scale.
Variables are expressed as mean±standard deviation (SD) and the p value.
The clinical and demographic characteristics of the population are shown in Table 1. The mean age of the population was 81.7±6.9 years, being higher in the group of non-survivors (83.5 vs. 81 years, p<0.001). The most prevalent comorbidities were hypertension (75.2%), dyslipidaemia (51.9%), diabetes (32%), atrial fibrillation (21%), and dementia (20.8%). The proportion of comorbidities such as dyslipidaemia, diabetes mellitus with target organ involvement, atrial fibrillation, dementia, degenerative neurological disease, and heart failure was significantly higher in the non-survivor group (p<0.001). However, although the proportion of cases with hypertension and COPD was also higher in the non-survivor group, no significant differences were found compared to survivors. Charlson index was high in all population, but more in the non-survivor group (6.3 vs. 5.3 points, p<0.001). Most patients (62,5%) were independent or with a middle level of dependence; nonetheless, the proportion of patients with a moderate or severe level of dependence was higher among non-survivors (34.2% vs 25.8% patients with moderate frailty, and 34.5% vs. 14.7% patients with severe frailty, respectively, p<0.001).
Regarding the degree of frailty, 174 patients (9.3%) were classified as very fit/fit (CFS 1−2); 795 patients (42.3%) as vulnerable (CFS 3−4); 529 patients (28.9%) were classified as moderately fragile (CFS 5−6) and 380 patients (20.2%) as severely fragile or terminal (CFS 7−9). The proportion of patients with moderate or severe frailty is significantly higher in non-survivors (p<0.001)
A higher proportion of patients in the non-survivor group had significantly higher levels of leukocytes, neutrophils, CRP, creatin, LDH, ferritin, and procalcitonin (Table 2) than survivors (p<0.001). Non-survivors also had higher lymphopenia and higher D-Dimer values, although this did not reach significance. Hypoxemia (Oxygen saturation <90%) was more frequent in non-survivor group (p<0.001). Radiological findings (condensations, bilateral infiltrates and pleural effusion) were significantly more frequent in the non-survivor group (p<0.001).
Laboratory, physical examination, and radiological findings in patients ≥70 years hospitalised because of COVID-19.
| Variables | All patients (n=1878) | Dead (n=527) | Alive (n=1351) | p |
|---|---|---|---|---|
| Temperature, 0C (mean±standard deviation) | 36.7±0.8 | 36.7±0.83 | 36,6±0.88 | 0.092 |
| Oxygen saturation <90% (mean±standard deviation) | 92.9±5.4 | 91.4±6.8 | 93.5±4.7 | <0.001 |
| Ches x-ray findings n (%) | ||||
| Pneumonic condensation n (%) | <0.001 | |||
| Unilateral | 172 (10.6) | 51 (12.2) | 121 (10.1) | |
| Bilateral | 312 (19.3) | 134 (32.1) | 178 (14.8) | |
| Interstitial lung infiltrates n (%) | <0.001 | |||
| Unilateral | 126 (7.8) | 23 (5.5) | 103 (8.6) | |
| Bilateral | 969 (59.9) | 299 (71.7) | 670 (55.8) | |
| Pleural effusion n (%) | <0.001 | |||
| Unilateral | 56 (3.5) | 27 (6.5) | 29 (2.4) | |
| Bilateral | 32 (2.0) | 16 (3.8) | 16 (1.3) | |
| Analytics (mean±standard deviation) | ||||
| Haemoglobin, g/dL | 12.7±2.0 | 12.4±2.2 | 12.8±2.0 | <0.001 |
| Leukocytes, 103/μL | 7665.3±4929.8 | 8871±6708 | 7196±3938 | <0.001 |
| Neutrophils, 103/μL | 5901.8±4333.5 | 7038±3888.4 | 5460±5144.0 | <0.001 |
| Lymphocytes, 103/μL | 1169.9±2657.6 | 1094.3±2729.9 | 1099.1±2629.5 | 0.444 |
| Platelets, 103/μL | 200±90 | 197±91 | 202±89 | 0.244 |
| C-reactive protein, mg/L | 90.1±85.6 | 115.7±101.2 | 80.9±76.6 | <0.001 |
| Creatinine, mg/dL | 1.3±1.0 | 1.6±1.2 | 1.2±0.9 | <0.001 |
| LDH, U/L | 357.5±230.2 | 407.4±302.7 | 338.7±193.0 | <0.001 |
| Ferritin, μg/L | 748.2±890.5 | 996.7±1197.8 | 663.5±739.8 | <0.001 |
| Albumin, g/dL | 3.4±3.5 | 3.2±0.5 | 3.5±-0.5 | <0.001 |
| Procalcitonin, ng/mL | 0.54±2.77 | 0.93±3.6 | 0.39±2.3 | 0.014 |
| D-dimer, ng/mL | 2376.9±14956.5 | 3749.9±27261.2 | 1869.4±5584.4 | 0.145 |
LDH, lactate deshydrogenase.
Variables are expressed as mean±standard deviation (SD) and p value.
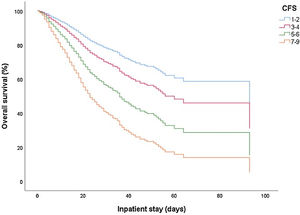
Frailty was associated with higher all-cause mortality after adjustment for age, sex, and comorbidities, showing worsening of clinical outcome with increased frailty (Table 3 and Fig. 1). The crude HR for time from hospital admission to mortality was 1.634 (95% CI 1.023−2.611; p0.04) for CFS 3−4; 2,887 (1,816−4,591; p<0.001) for CFS 5−6 and 5,557 (3,493−8,841; p<0.001) for CFS 7−9, all compared to CFS 1−2. The adjusted HR was 1.458 (95% CI 0.903−2.355; p0.001) for CFS 3−4; 2,344 (1,437−3,823; p<0.001) for CFS 5−6 and 3,694 (2,155−6,330; p<0.001) for CFS 7−9. Elevated CRP, lymphopenia, neutrophilia, hypoxemia, and bilateral chest X-ray infiltrates were also significantly associated with mortality (p<0.001). Severe dependence was also significantly associated with mortality in the univariate analysis with a crude HR of 2.409 (1.984−2.926; p<0.001); however, in the multivariate analysis this association was not significant adjusted HR 1.184 (0.879−1.595; p0.266).
Prognostic factors for in-hospital survival. Univariate and multivariate analysis.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | |
| CFS 1−2 | Reference | |||||
| CFS 3−4 | 1.634 | 1.023−2.611 | 0.040 | 1.458 | 0.903−2.355 | 0.123 |
| CFS 5−6 | 2.887 | 1.816−4.591 | <0.001 | 2.344 | 1.437−3.823 | <0.001 |
| CFS 7−9 | 5.557 | 3.493−8.841 | <0.001 | 3.694 | 2.155−6.330 | <0.001 |
| C-reactive protein ≥ 80mg/l | 2.196 | 1.844−2.613 | <0.001 | 1.835 | 1.530−2.201 | <0.001 |
| Lymphocytes < 0.800×103/μL | 1.829 | 1.537−2.175 | <0.001 | 1.706 | 1.418−2.051 | <0.001 |
| Neutrophils ≥ 7.5×103/μL | 1.676 | 1.412.990 | <0.001 | 1.503 | 1.256−1.799 | <0.001 |
| SatO2 <90% | 1.665 | 1.371−2.020 | <0.001 | 1.492 | 1.218−1.827 | <0.001 |
| Bilateral interstitial lung infiltrates on x-ray | 1.559 | 1.255−1.936 | <0.001 | 1.375 | 1.092−1.732 | 0.007 |
| Severe dependency | 2.409 | 1.984−2.926 | <0.001 | 1.184 | 0.879−1.595 | 0.266 |
| Charlson index | 1.154 | 1.115−1.194 | <0.001 | 1.067 | 1.023−1.113 | 0.003 |
| Age, years | 1.057 | 1.044−1.070 | <0.001 | 1.032 | 1.018−1.047 | 0.000 |
| Female Sex | 1.119 | 0.857−1.212 | 0.828 | 0.989 | 0.821−1.191 | 0.906 |
Our study determines the importance of early detection of frailty in older patients hospitalised due to COVID-19, considering it as main risk factor associated with adverse outcomes compared to other risk factors already identified in previous studies. The main predictors of poor prognosis during the acute phase of infection have been described in previous studies. However, most of them have been performed in the general population and only some have targeted the older patient population or have evaluated the degree of frailty.17
Older age is associated with increased mortality but is not sufficient on its own for risk stratification in patients with COVID-19 and is subject to ethical controversy.11 Pre-admission functional status plays an important role in the evolution of this patient profile, especially those with a moderate-severe degree of frailty who are at higher risk of adverse outcomes. This relationship between frailty and increased mortality has been extensively studied in other diseases. In COVID-19 an increasing number of studies have identified frailty as one of the main prognostic factors of the disease, but more evidence is still needed.
Previously, the SEMI-COVID registry19 had identified the main predictors of poor prognosis in very older patients (>80 years) hospitalised due to COVID-19 infection and this study was the first one to identify the prognostic significance of pre-admission clinical status on the outcome of geriatric patients, finding that a severe degree of dependency (defined as a Barthel Index ≤60) was an independent predictor of mortality. Nonetheless, one of the main limitations of this study was that it did not evaluate frailty. Advanced age, severe level of dependency, male sex, certain laboratory, and chest X-ray abnormalities were identified as the main predictors of poor clinical outcome in this population. In contrast, comorbidities were not associated with increased mortality. Our data reflect concordance with the results of this study except for some aspects. No association was found between intrahospital survival and sex or level of severe dependency. However, a high degree of comorbidity (defined by Charlson index ≥4) was associated with lower intrahospital survival.
Studies assessing frailty in COVID-19 such as the COPE study,17 and others27 demonstrated that frailty was associated with mortality and longer hospital stay, showing a worsening of clinical outcome with increasing frailty. Our results do not only confirm these assertions, but also establish the presence of moderate-severe frailty as the main prognostic factor independently associated with all-cause hospital survival compared to the other factors identified in previous studies. In addition, there is a direct relationship between a higher degree of frailty and lower intrahospital survival.
The assessment of the degree of frailty was carried out using the quantitative method CFS, which is the most widely used in other studies.28,29 The CFS is a reliable and potentially useful screening tool to identify frailty. It is also easily applicable even in a situation of limited human resources and increasing demand for medical services,30 as it was the case in the COVID-19 pandemic. Other frailty measures are available for the assessment of frailty in hospitalised patients but are either more time consuming to apply or rely on routinely collected data to score frailty.31 The National Institute for Health and Care Excellence (NICE) published in 2020 the COVID-19 rapid guidelines for adult critical care, recommending the use of the CFS in patients aged 65 and over to aid clinical decision making and avoid age discrimination.32
Therefore, the early assessment of frailty represents a valuable opportunity to provide higher quality care to older adults with COVID-19. Early detection and careful monitoring of frailty can alert us to the possibility of adverse outcomes and help us to provide appropriate clinical management in this patient profile. The initial approach to these patients should incorporate an appropriate functional assessment including the evaluation of the degree of frailty. In addition, we would like to emphasise the importance of the use of the CFS scale as a predictor of unfavourable events in this population.
This study has some limitations. First, this is a retrospective series focusing on hospitalised patients. Since these patients had more severe disease and a higher mortality rate, our data may overestimate the overall mortality in the totality of adults over 70 years of age with COVID-19. Second, as a retrospective cohort study, the data were collected by a large number of investigators, which could have led to heterogeneity in data entry and validation.
ConclusionIn older patients hospitalised due to COVID-19 infection, the degree of frailty is the main predictor of intrahospital survival, showing that it increases the risk of all-cause mortality after adjustment for age, comorbidities, and other prognostic factors related to the severity of the infection. These findings highlight the need for early detection of frailty using clinical scales, which is of vital importance in establishing a prognosis in this population.
FundingThe authors declare that they have not received funding for conducting this study.
Conflicts of interestThe authors declare that they do not have any conflicts of interest.
We would like to thank Ms. Claudia Corazza González for her help with the final English-language version.