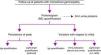
We present guidelines from the Immunochemistry group of the Spanish Society for Immunology that are designed to provide a practical tool for the diagnosis and follow-up of monoclonal gammopathies. We review the clinical and analytical features of various monoclonal gammopathies, international consensus guidelines and techniques used to detect and follow-up monoclonal components.
Se presenta una guía elaborada por el grupo de Inmunoquímica de la Sociedad Española de Inmunología con el objetivo de proporcionar una herramienta práctica para el diagnóstico y seguimiento de las gammapatías monoclonales.
Se revisan las características clínicas y analíticas de los diferentes tipos de gammapatía monoclonal, las guías de consenso internacionales y las técnicas utilizadas para la detección y seguimiento del componente monoclonal.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<