COVID-19 shows different clinical and pathophysiological stages over time. The effect of days elapsed from the onset of symptoms (DEOS) to hospitalization on COVID-19 prognostic factors remains uncertain. We analyzed the impact on mortality of DEOS to hospitalization and how other independent prognostic factors perform when taking this time elapsed into account.
MethodsThis retrospective, nationwide cohort study, included patients with confirmed COVID-19 from February 20th and May 6th, 2020. The data was collected in a standardized online data capture registry. Univariate and multivariate COX-regression were performed in the general cohort and the final multivariate model was subjected to a sensitivity analysis in an early presenting (EP; <5 DEOS) and late presenting (LP; ≥5 DEOS) group.
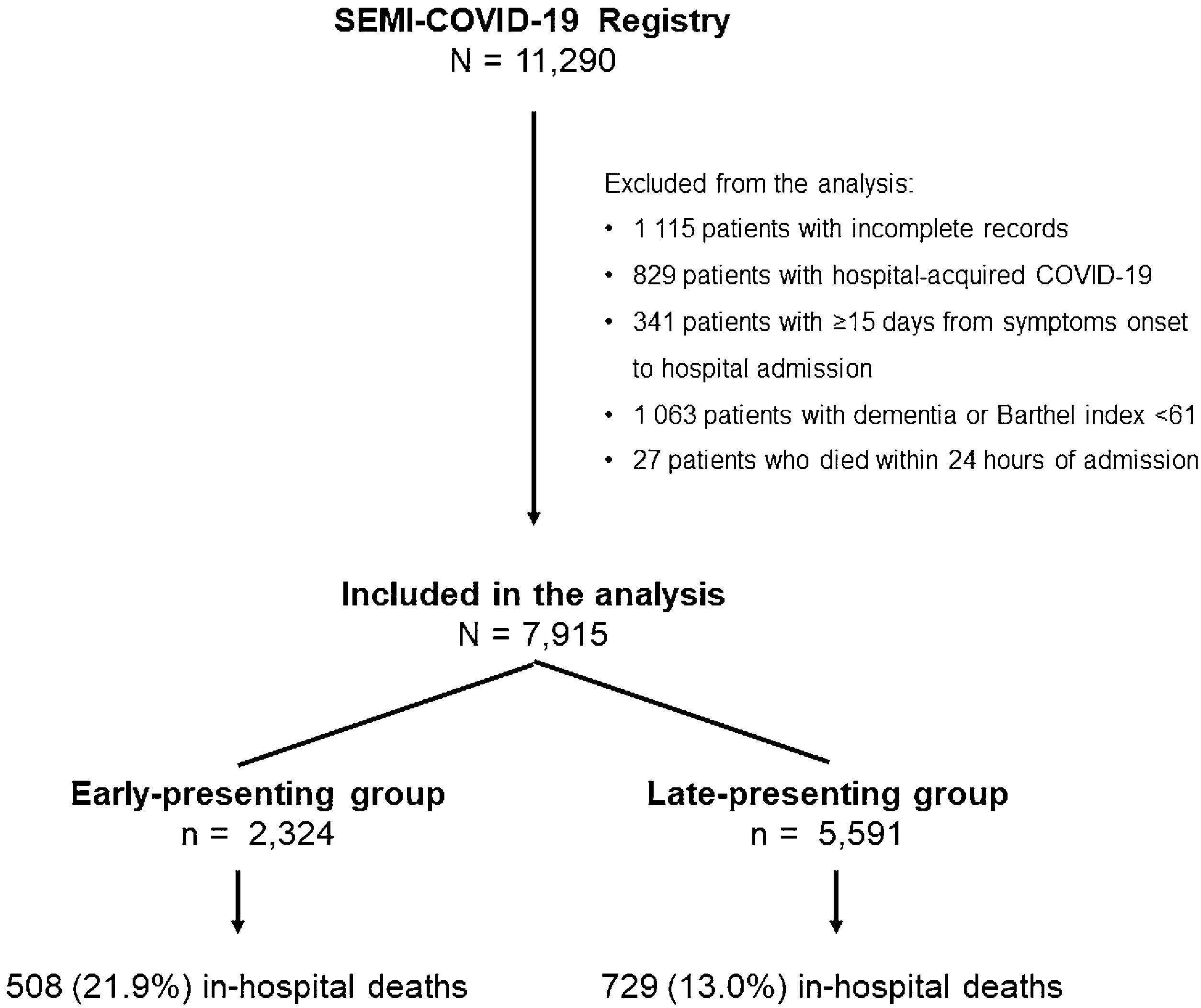
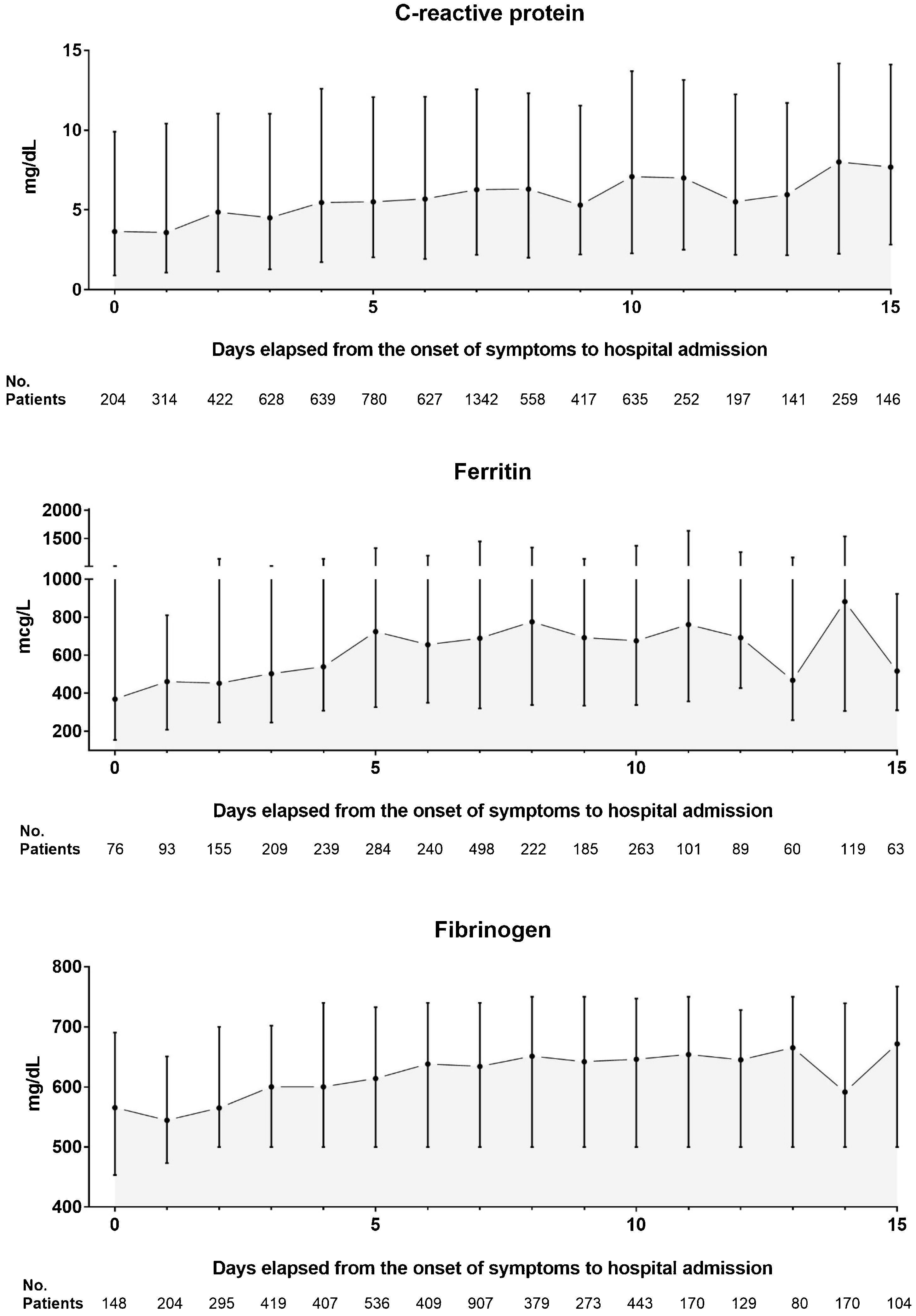
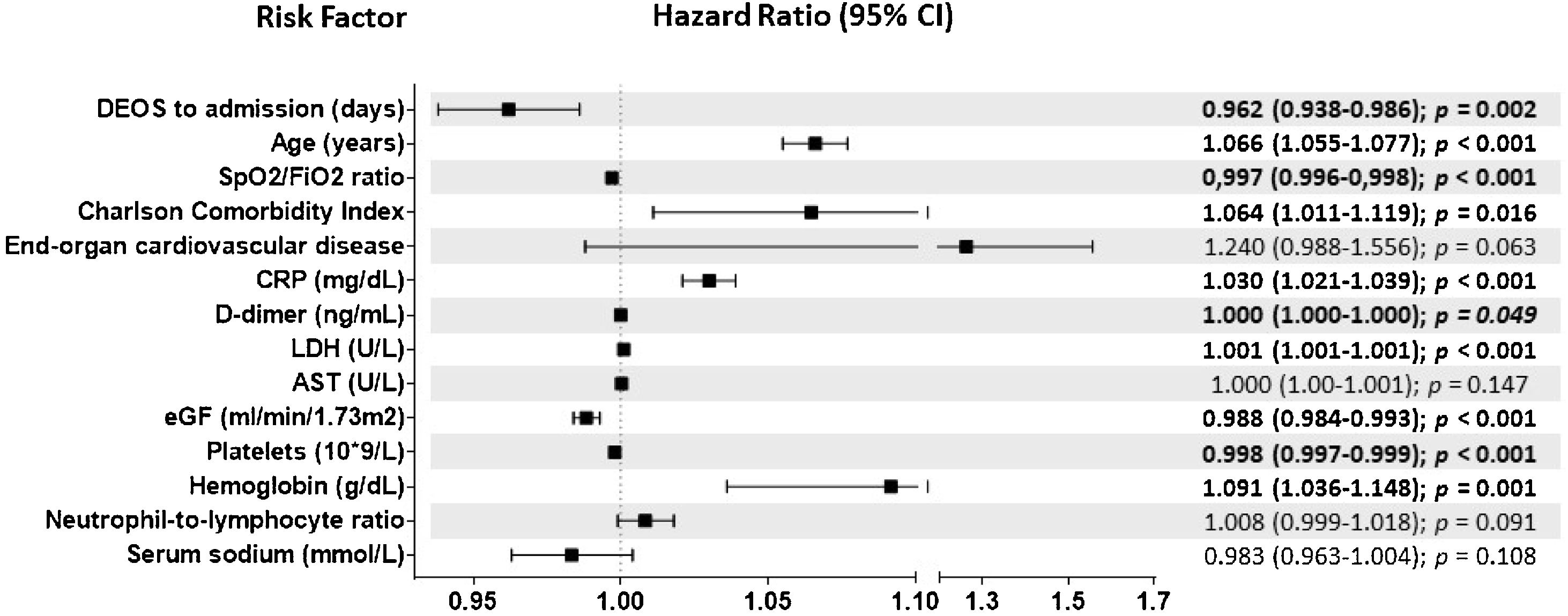
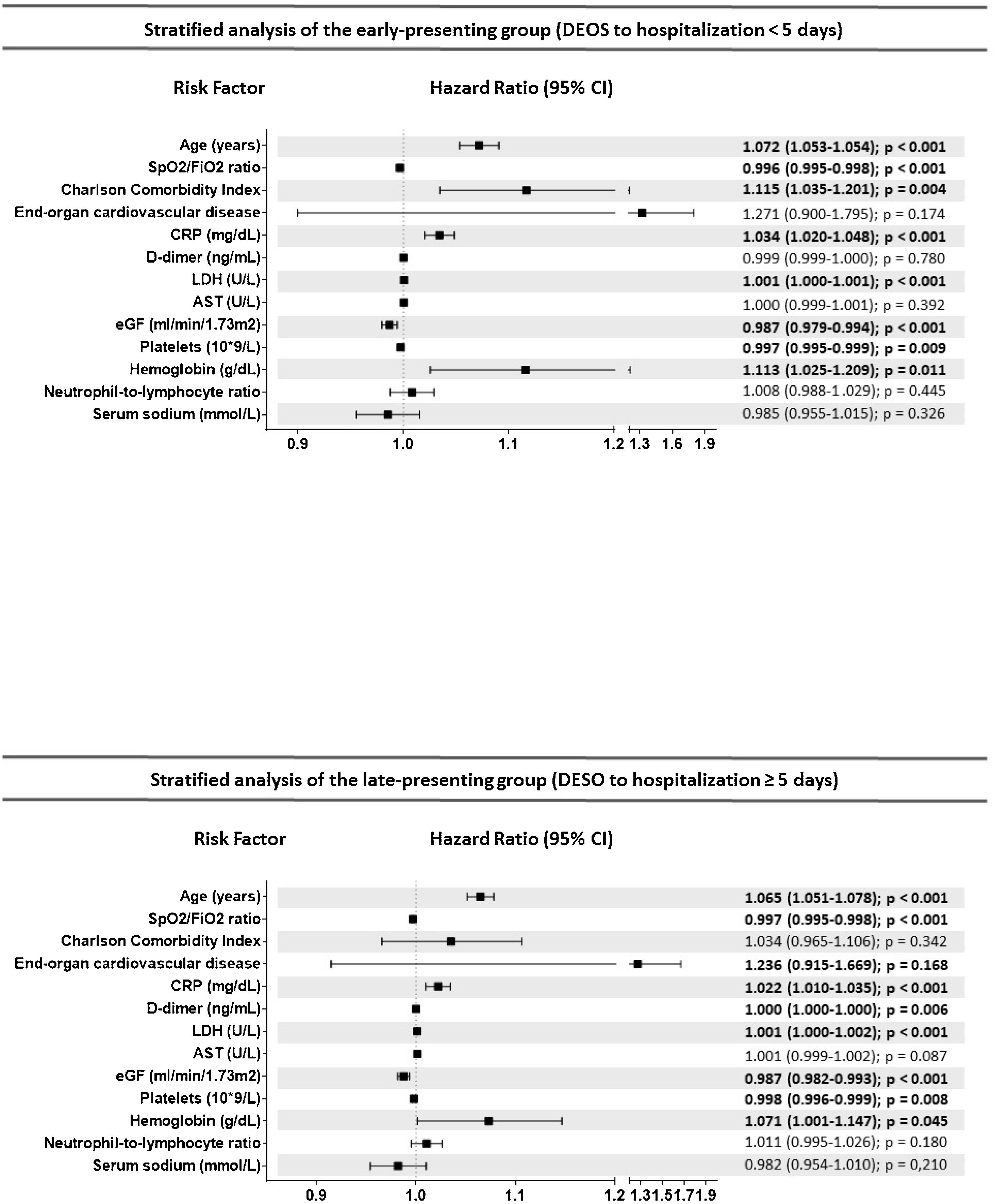
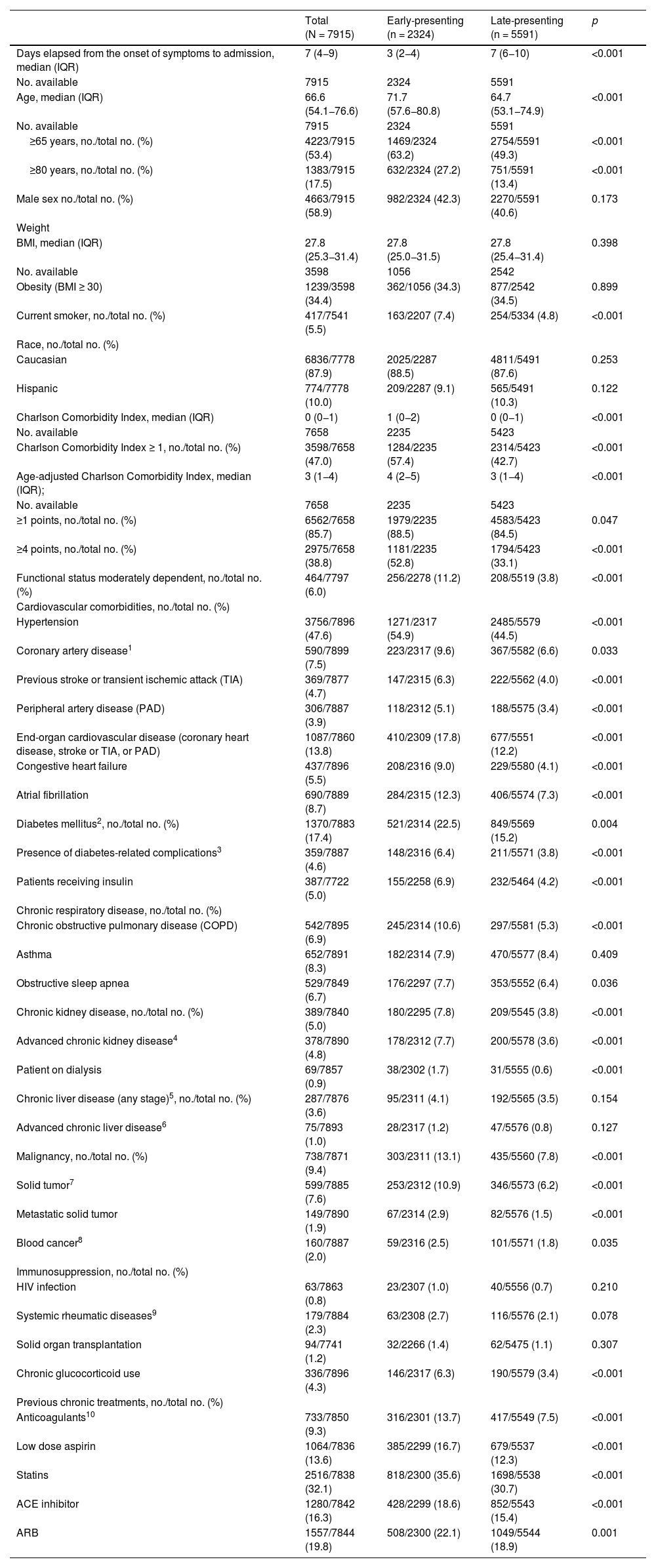
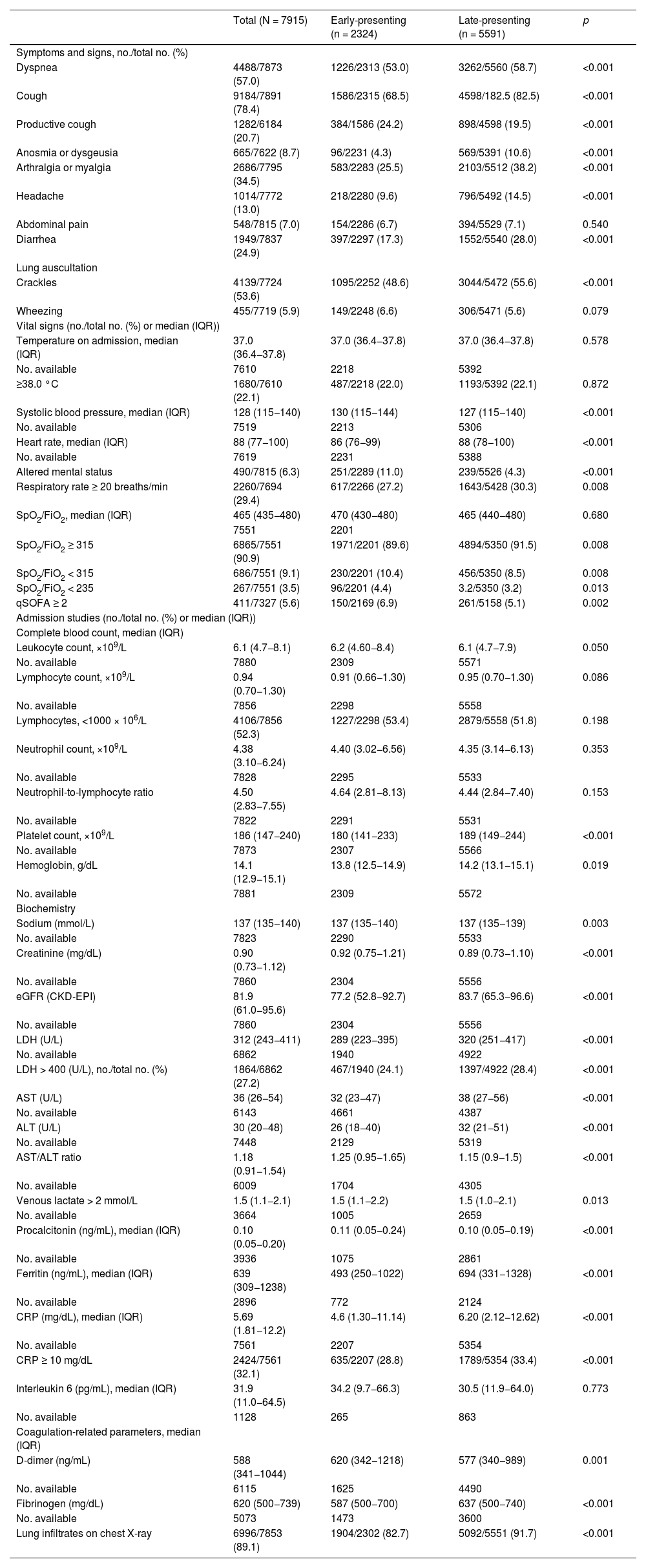
Results7915 COVID-19 patients were included in the analysis, 2324 in the EP and 5591 in the LP group. DEOS to hospitalization was an independent prognostic factor of in-hospital mortality in the multivariate Cox regression model along with other 9 variables. Each DEOS increment accounted for a 4.3% mortality risk reduction (HR 0.957; 95% CI 0.93–0.98). Regarding variations in other mortality predictors in the sensitivity analysis, the Charlson Comorbidity Index only remained significant in the EP group while D-dimer only remained significant in the LP group.
ConclusionWhen caring for COVID-19 patients, DEOS to hospitalization should be considered as their need for early hospitalization confers a higher risk of mortality. Different prognostic factors vary over time and should be studied within a fixed timeframe of the disease.
La COVID-19 muestra diferentes fases clínicas y fisiopatológicas a lo largo del tiempo. El efecto de los días transcurridos desde el comienzo de los síntomas (DTCS) hasta la hospitalización sobre los factores pronósticos de la COVID-19 sigue siendo incierto. Analizamos el impacto en la mortalidad de los DTCS hasta la hospitalización y cómo se comportan otros factores pronósticos independientes al tener en cuenta dicho tiempo transcurrido.
MétodosEn este estudio de cohortes nacional retrospectivo se incluyó a pacientes con COVID-19 confirmada entre el 20 de febrero y el 6 de mayo de 2020. Los datos se recopilaron en un registro normalizado de captura de datos en línea. Se realizó una regresión de Cox uni y multifactorial en la cohorte general y el modelo multifactorial final se sometió a un análisis de sensibilidad en un grupo de presentación precoz (PP; <5 DTCS) y otro de presentación tardía (PT; ≥5 DTCS).
ResultadosEn el análisis se incluyó a 7915 pacientes con COVID-19, 2324 en el grupo de PP y 5591 en el de PT. Los DTCS hasta la hospitalización fueron un factor pronóstico independiente de mortalidad intrahospitalaria en el modelo de regresión de Cox multifactorial junto con otras nueve variables. Cada incremento en un DTCS supuso una reducción del riesgo de mortalidad del 4,3 % (RRI = 0,957; IC del 95 %, 0,93—0,98). En cuanto a las variaciones de otros factores predictivos de la mortalidad en el análisis de sensibilidad, únicamente el índice de comorbilidad de Charlson siguió siendo significativo en el grupo de PP, mientras que únicamente el dímero D lo siguió siendo en el grupo de PT.
ConclusionesAl atender a pacientes con COVID-19 hay que tener en cuenta los DTCS hasta la hospitalización porque la necesidad de hospitalización precoz confiere un mayor riesgo de mortalidad. Los diferentes factores pronósticos varían con el tiempo y deberían estudiarse dentro de un marco temporal fijo de la enfermedad.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<