This updated version of the Spanish Society for Research in Osteoporosis and Mineral Metabolism (SEIOMM) osteoporosis guides incorporate the most relevant information published in the last 7 years, since the 2015 guides, with imaging studies, such as vertebral fracture assessment and bone trabecular score analysis. In addition, therapeutic advances include new anabolic agents, comparative studies of drug efficacy, and sequential and combined therapy. Therefore, therapeutic algorithms are also updated.
Esta versión actualizada de las guías de osteoporosis de la Sociedad Española de Investigación en Osteoporosis y Metabolismo Mineral (SEIOMM) incorpora la información más relevante publicada en los últimos 7 años, desde las guías de 2015, con estudios de imagen, como la valoración de la fractura vertebral y el análisis del índice trabecular óseo. Además, los avances terapéuticos incluyen los nuevos fármacos anabólicos, los estudios comparativos de la eficacia de los fármacos y la terapia secuencial y combinada. Por ello se actualizan también las recomendaciones de los tratamientos.
Seven years have passed since the most recent version of the Osteoporosis Guidelines of the Spanish Society for Bone Research and Mineral Metabolism (SEIOMM) was drawn up, using the standard methodology of evidence-based medicine.1 This update incorporates information released since then. The full text is available in annex II.
MethodsA group of experts (see annex I) reviewed each section to incorporate the new findings published in recent years. The new text was disseminated to other interested entities (including SEIOMM partners, patient associations, the Spanish Agency for Medicines and Health Products, and pharmaceutical industries) to provide input to the document, which was subsequently analysed by the group of experts. Osteoporosis in postmenopausal women was analysed first, followed by osteoporosis in men and glucocorticoid-induced osteoporosis.
Assessment of patients at risk of osteoporosisClinical risk factors for fractureThe main risk factors are shown in Table 1. After suffering a first fracture, the greatest risk of suffering a new fracture occurs in the subsequent two years, especially if the first fracture was vertebral.2 This phenomenon led to formulating the concept of “imminent risk” of fracture.
Diseases and treatments that are risk factors for osteoporosis.
| Factors clearly associated with osteoporosis |
| Advanced age |
| Female sex |
| Personal history of fracture |
| Family history of hip fracture |
| Increased risk of falls |
| Diseases |
| Hypogonadism |
| Early menopause, amenorrhea |
| Anorexia nervosa |
| Malabsorption |
| Rheumatoid arthritis |
| Diabetes (particularly type 1) |
| Immobilisation |
| Cushing's disease |
| Treatments |
| Glucocorticoids |
| Aromatase inhibitors |
| Gonadotropin-releasing hormone agonists (and other androgen deprivation treatments in men) |
| Other factors associated with less consistency |
| Hyperparathyroidism. hyperthyroidism |
| Calcium deficiency |
| Vitamin D deficiency |
| Drugs and toxic |
| Selective serotonin reuptake inhibitors |
| Proton-pump inhibitor |
| Anticonvulsants |
| Antiretrovirals |
| Alcohol, tobacco |
X-ray absorptiometry (DXA), which quantifies bone mineral density (BMD), is commonly used to estimate fracture risk. The diagnosis of osteoporosis is established with a T score <−2.5 in any of the following locations: lumbar spine, total hip, or femoral neck (Table 2).
WHO diagnostic criteria for osteoporosis.
| Normal: BMD T≥−1 |
| Osteopenia or low bone mineral density: BMD T<−1 and >−2.49 |
| Osteoporosis: BMD T≤−2.5 |
| Severe osteoporosis: BMD T≤−2.5+fracture |
BMD: bone mineral density; T (T-score or T index): comparison with the BMD value reached in a young reference population.
In premenopausal women and men under 50 years, the use of Z scores is recommended, with Z≤−2.0 considered “low BMD for chronological age.”
The trabecular bone score may improve the prediction of fracture risk.
In general, DXA is recommended when risk factors are strongly associated with osteoporosis or fractures (Table 1).
Radiography is essential for identifying fractures. In the case of the vertebralfractures, the diagnosis requires a decrease of at least 20–25% in height. In some cases, imaging based on DXA (i.e., vertebral fracture assessment, VFA) may be an alternative.
Study protocol—bone turnover markersA complete blood count and biochemical analysis should be carried out (kidney and liver function, calcium, albumin, phosphorus, alkaline phosphatase, thyrotropin, 25-hydroxyvitamin D [25OHD], proteinogram and calciuria). The suitability of determining parathyroid hormone (PTH) and bone turnover markers (BTM) is a subject of debate. Other studies should be performed in young patients to rule out secondary causes of osteoporosis (e.g., hypercortisolism, celiac disease, and systemic mastocytosis).
DXA and evaluation of possible vertebral fractures will almost always be necessary.
Together with other risk factors, BTMs can aid in identifying patients with a higher risk of fracture and, (above all) they help early assessment of the response to treatment. The most widely used are the carboxyterminal telopeptides of type I collagen (s-CTX, Serum C-telopeptide cross-link type 1 collagen) and the amino-terminal peptides of type I procollagen (procollagen type 1 N-terminal propeptide).
Risk prediction toolsA combination of clinical data and DXA is useful to assess fracture risk. Several instruments have been developed for this purpose, including FRAX, the Garvan Medical Research Institute scale, and the QFracture Index. They have a similar discriminatory capacity and are only moderately efficient. FRAX is the most widespread. Unfortunately, its adaptation to the epidemiology of fractures in Spain has been inadequate and underestimates the risk of major osteoporotic fractures.
Available treatments for postmenopausal osteoporosisNon-pharmacological interventionsA balanced diet should be maintained, with a contribution of 1–1.5g/kg of protein, regular physical exercise, and avoiding tobacco and excessive alcohol consumption. Fall prevention programmes and hip protectors may be helpful in some cases.
Calcium and vitamin DPatients treated with drugs for osteoporosis should have an adequate intake of calcium and vitamin D3,4 to attain serum levels of 25OHD>25–30ng/mL. The generally recommended dose of vitamin D is 800–1200IU/d (or weekly or monthly equivalent). If calcifediol is used, 0.266 micrograms are given every 15–30 days. Calcium intake should be 1000–1200mg/day, preferably through diet and supplements if needed.
Drugs not indicated in osteoporosisCalcitonin, strontium ranelate, PTH 1–84, isoflavones, phytoestrogens, and tibolone are not indicated for the treatment of osteoporosis. Thiazides can be used to control hypercalciuria.
Oestrogen therapyAlthough oestrogen therapy effectively prevents fractures, its possible side effects have prevented it from being recommended as an osteoporosis treatment, except in cases of early menopause or when other alternatives are not available.
Selective oestrogen receptor modulatorsSelective oestrogen receptor modulators (SERMs) increase spinal BMD. Raloxifene and bazedoxifene reduce vertebral fracture risk by 40% but do not influence nonvertebral fractures.5 Its main complication is an increased risk of venous thromboembolic disease.
BisphosphonatesAlendronateAlendronate at 70mg/week reduces vertebral, nonvertebral, and hip fractures by around 45%, 25–30%, and 45–55%, respectively.6 Most clinical trials have included a treatment period of 3–5 years. However, a more prolonged administration may sometimes be recommended.
RisedronateAccording to recent meta-analyses, risedronate reduces the risk of all fractures (vertebral 39%, hip 27% and non-vertebral 22%).5 It is administered in doses of 35mg weekly or 75mg two consecutive days per month. A weekly gastro-resistant formulation does not require administration on an empty stomach.
IbandronateThis agent is less effective than other bisphosphonates (BPs) and does not appear to reduce nonvertebral fractures.
ZoledronateZoledronate at 5mg/year intravenously reduces vertebral, non-vertebral and hip fractures by 70%, 25%, and 40%, respectively.7 A network meta-analysis found no differences between the BPs in terms of fracture prevention, while in another two, zoledronate was more effective than other BPs.
Adverse effects of bisphosphonatesBPs are generally well tolerated. In some patients, oral BPs can cause esophagitis. They should be avoided in patients with difficulty swallowing or Barrett's oesophagus. Acute-phase reaction or self-limited flu-like symptoms are common after the first dose of zoledronate. BPs are not recommended in patients with a glomerular filtration rate (GFR) ≤30mL/min. Intravenous BPs can cause hypocalcaemia, especially in patients with renal failure or insufficient intake of vitamin D or calcium.
Osteonecrosis of the jaws (ONJ) is rare but potentially severe. The risk in patients treated with BP for osteoporosis is very low (1/1500–1/100,000 patient-years). It is related to the state of oral health (periodontitis) and dental procedures.
Atypical fractures of the femur (AFF) occur in 1–2 cases per 10,000 patients treated with BP. The risk increases with exposure time; however, this risk is very low compared to the risk of osteoporotic fractures. For each AFF that could appear, some 270 clinical fragility fractures are prevented, including 70 hip fractures.8
DenosumabDenosumab reduces the risk of vertebral, non-vertebral, and hip fractures by around 70%, 20%, and 40%, respectively.9
It is generally well tolerated. The risks of AFF and ONJ are very low, around 1/10,000 and 1/2000 patients/year, respectively. Denosumab can be used in patients with kidney failure, even those on dialysis. An adequate supply of calcium and vitamin D must be ensured to avoid hypocalcaemia.
After discontinuation, an increase in bone turnover markers (BTM) and a loss of BMD gained are observed. In some patients, this phenomenon is associated with multiple vertebral fractures.
PTH 1–34 (teriparatide)Teriparatide exerts a bone-forming effect and reduces vertebral fracture risk by 65% and non-vertebral fractures by 50%. A meta-analysis did not show a significant reduction in hip fractures, but another three concluded that it reduced these fractures by 56–65%. It was shown to be more effective than risedronate in women with severe osteoporosis.10 Several biological analogues and biosimilars are marketed.
AbaloparatideAbaloparatide reduces vertebral and non-vertebral fractures. It is approved in the US but not in Europe.
RomosozumabRomosozumab is a sclerostin-neutralising antibody with dual anabolic and antiresorptive effects.
According to several meta-analyses,5,11 this agent reduces vertebral (66–73%), non-vertebral (33%), and hip (56%) fractures. In women with severe osteoporosis, a cycle of romosozumab provided additional benefits to alendronate.12
Romosozumab is generally well tolerated; however, in some studies, a small increase in cardiovascular events was described (1.3% vs 0.9%); therefore, it is contraindicated in patients with a history of myocardial infarction or cerebrovascular accident and should be considered carefully in those with multiple cardiovascular risk factors.
Vertebroplasty and kyphoplastyAlthough many noncontrolled studies have shown a marked analgesic effect, randomised clinical trials have provided conflicting results for vertebroplasty and kyphoplasty. Thus, they are not routinely recommended.
They can be considered in patients with fractures less than 6 weeks old and severe pain despite medical treatment and in patients with fractures from 6 weeks to a year of evolution and persistent pain that responds poorly to analgesics if they show signs of oedema on MRI.
Start and follow-up of treatmentThe decision to commence treatmentIn general, patients with some of these characteristics should be treated:
- 1.
One or more fragility fractures, especially the vertebrae, hip, humerus, and pelvis (regardless of BMD).
- 2.
BMD<−2.5T score in the lumbar spine, femoral neck, or total hip.
- 3.
BMD in the “osteopenia” range (particularly if T is <−2.0) together with factors strongly associated with fracture risk (e.g., hypogonadism or early menopause, treatment with glucocorticoids or antiestrogens).
Some situations require an individualised assessment of the clinical characteristics. In young women with only slightly low BMD and no fractures or other risk factors, delaying treatment can be considered because the absolute risk of fracture is low. By contrast, the coincidence of several important risk factors may lead to earlier treatment consideration. Scales that help estimate fracture risk (e.g., FRAX) may be helpful, although their validity in the Spanish population is limited.
Control of the therapeutic responseIf necessary, adherence to treatments can be monitored using BTMs, whose changes predict therapeutic response.
The beneficial effect of the treatment is confirmed by the evolution of BMD and the absence of new fractures. A change of treatment may be considered due to a possible inadequate response if two new fractures appear during treatment or two of the following events occur: a new fracture, a significant decrease in BMD (e.g., 4−5%), or a decrease of the BTM less than the minimum significant change (approximately 25%).
Duration of treatmentSeveral aspects must be considered. Although the treat-to-target strategy is theoretically attractive, the aims to be achieved in treating osteoporosis are not well defined, limiting its practical application. For some experts, the absence of new fractures and an increase in BMD would be the most appropriate. Various experts have recommended a T score greater than −2.0 or −2.5 as a target, especially in the hip.
Several studies demonstrated the persistence of the effect by maintaining zoledronate for 6 years or alendronate or denosumab for 10 years. However, side effects (particularly ONJ and AFF) may increase with the duration of treatment. Therefore, it is recommended to reassess patients treated with BP at 3 (zoledronate) or 5 years (oral BP) and those treated with denosumab at 5–10 years.
Treatment should be continued (with the same drug or with another) if any of the following circumstances occur:
- a.
BMD at the femoral neck <−2.5T.
- b.
The appearance of fragility fractures in the 3–5 years before evaluation.
- c.
Some experts also recommend continuing treatment if the patient has a history of hip or vertebral fracture at some point in life.
If none of these circumstances occurs, treatment with BP can be withdrawn, at least temporarily (“therapeutic holidays”): for risedonate, 1 year; for alendronate, 2 years; and for zoledronate, 3 years. In the case of denosumab, temporary interruptions should not be considered.
Sequential and combined treatmentBisphosphonates after denosumabAfter discontinuation of denosumab, bone turnover increases beyond baseline values (“rebound effect”). This is associated with a rapid decrease in bone mass gained and vertebral fractures in some cases. To avoid this occurrence, a powerful BP should be administered.13 The first dose of zoledronate should be prescribed when denosumab is discontinued (i.e., 6 months after the last dose) and repeated when elevated BTMs are detected, generally at 6 or 12 months.
If the BTMs cannot be measured, the administration of zoledronate should be repeated 6 and 12 months after the previous administration, and the need for new doses should be individually considered. In patients who have received denosumab for fewer than 2.5 years, alendronate can be used instead of zoledronate.
Antiresorptive agents after anabolicsAfter finishing treatment with anabolic drugs such as teriparatide or romosozumab, the administration of a BP or denosumab is recommended.
Anabolic drugs after antiresorptive drugsThe previous use of BP slightly reduces the BMD gain obtained with teriparatide. Therefore, the preferred sequence is first an anabolic drug and then an antiresorptive. However, previous treatment with BP does not contraindicate the administration of anabolics. Of course, teriparatide should not be started as the only treatment in the months after stopping denosumab, given the risk of the accelerated loss of bone mass.
Combined treatmentThere are not enough trials to recommend it routinely. The combination of teriparatide with denosumab or zoledronate may be considered in particularly severe cases with a high risk of hip fracture.
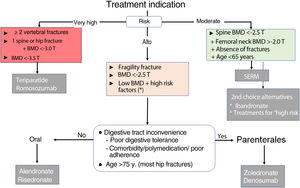
Therapeutic decision algorithmsInitial treatment (choice of drug, Fig. 1)The main criterion for the choice of the initial drug is the level of fracture risk:
- 1.
Moderate risk. This level corresponds to the risk profile of a woman under 65 years of age, with no history of fracture, moderately low BMD in the spine (T score between −2.5 and −3.0) and preserved in the hip (T>−2). In this situation, it is advisable to use a SERM and thus delay the use of drugs with possible long-term adverse effects. Ibandronate and other antiresorptives are alternative options.
- 2.
High risk. This level corresponds to most of the cases. Alendronate, risedronate, zoledronate, and denosumab are indicated. Oral BPs are preferred in patients without inconveniences for oral administration (digestive problems, polypharmacy, adherence) and preferably under 75 years of age.
- 3.
Very high risk. This level corresponds to women with (a) 2 or more vertebral fractures, or equivalent situation (e.g., vertebral and hip fracture); or (b) very low BMD (T<−3.5); or (c) vertebral or hip fracture together with T<−3.0. There may be other situations (difficult to systematise) in which clinical factors determine very high fracture risk and require individualised consideration. For this level of risk, bone-forming drugs are preferable.
Figure 1.Algorithm for selection of initial treatment in postmenopausal osteoporosis.
BMD: bone mineral density; SERM: selective oestrogen receptor modulator.
* Especially if T≤−2 and factors strongly associated with fracture risks, such as hypogonadism, early menopause, or treatment with glucocorticoids or sex hormone antagonists. These general criteria may need to be adapted based on other clinical determinants of fracture risk, the characteristics of individual patients, and their preferences.
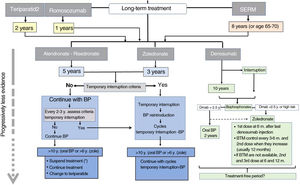
Romosozumab should only be given for 1 year and teriparatide for 2 years. SERMs can be continued for 8 years or until the patient reaches 65–70 years. Then it will be necessary to administer another antiresorptive, BP or denosumab.
Long-term treatment continuation algorithm.
BP: bisphosphonates; SERM: selective oestrogen receptor modulators; ROM: bone turnover markers.
* There are not enough data to establish a recommendation after that treatment time, so the possible options are listed before a decision that must be individualised.
The continued use of denosumab is recommended for 5–10 years. There is no information available regarding more prolonged use, so at that time, continuing treatment or discontinuing it should be carefully considered. In any case, a BP should be administered subsequently.
After the initial treatment cycle with BP, an interruption can be considered if the requirements to start a “therapeutic holiday” are met (see the end of section 3). No quality studies are available to guide decision making after 10 years.
Male osteoporosisMost of the drugs have shown gains in BMD like those observed in women, suggesting that their efficacy for fractures is also similar. Alendronate, risedronate, and zoledronate have been shown to reduce vertebral fractures in men. Denosumab has been shown to increase BMD in men and reduce fracture risk in those undergoing androgen deprivation. Teriparatide has also shown beneficial effects in men.14 For this reason, a strategy for choosing a drug like that for women should be proposed for men: (a) risedronate or alendronate (although the latter is not approved in Spain for treating male osteoporosis) as the treatment of choice for most patients; (b) zoledronate or denosumab in the elderly or when the oral route is not advisable; and (c) teriparatide in very high-risk patients.
Glucocorticoid-induced osteoporosisThe drugs of choice are BPs. If there are vertebral fractures, preferential treatment with teriparatide is justified due to its greater anti-fracture effect.15 Calcium and vitamin D should also be given.
Postmenopausal women and men older than 50 years who are to receive doses of ≥5mg/d of prednisone for >3 months should be treated. In premenopausal women and men <50 years of age, treatment is indicated only if there are previous fractures, BMD is low, or the dose of glucocorticoids is very high (>30mg/d). Denosumab is an alternative when other antiresorptive agents cannot be used.
FundingThis guide has been produced with the administrative support of the SEIOMM, without public or private funding.
Conflicts of interestThe individual conflicts of interest of the authors who have them are:
Antonio Cano: conference fees (Gedeon Richter) and advisory boards (Theramex). Cristina Carbonell Abella: conference fees (Amgen, UCB, Stada, Theramex, Angelini, Gebro) and travel grants (Amgen, Rubio). Enrique Casado Burgos: conference fees (Ucb, Gedeon-Richter, Stada, Grunenthal, Lilly, Amgen, Theramex, Gebro, Italfarmaco, Angelini), travel grants (Lilly, Amgen, Stada) and advisory boards (Theramex, Bayern, Gp-Pharm, Gebro, Gedeon-Richter, Stada). Manuel Ciria Recasens: conference fees (Grunenthal, Angelini, Gedeon Richter, Theramex, Rubio, Gebro Pharma) and travel grants (Amgen, Lilly, Rubio). Javier del Pino Montes: conference fees (Gedeon-Ritcher, Grünenthal, Ucb) and travel grants (Amgen). Luis Miguel del Rio Barquero: conference fees (Amgen, Gedeon-Richter). Manuel Díaz Curiel: travel grants (Rubio). Adolfo Díez Pérez: stocks, employee (Active Life Sci) and conference fees (Amgen, Lilly, Theramex). Alberto García Vadillo: conference fees (Lilly. Amgen. Gebro-Pharma. Theramex) and travel grants (UCB. Lilly. Amgen). Carlos Gómez Alonso: stocks (Faes), conference fees (Stada, Grünenthal, Amgen, UCB), travel grants (Amgen), research fellowship (Stada, Kiowa Kirin, FAES) and advisory boards (Amgen, Kiowa Kirin). Jesús González Macías: conference fees (Amgen-UCB, Gedeon Richter, Menarini, Theramex), travel grants (Lilly) and research fellowship (Faes). Nuria Guañabens: conference fees (Eli Lilly, Amgen, UCB), travel grants (Eli Lilly, Amgen, UCB) and advisory boards (Amgen, UCB). Esteban Jodar Gimeno: stocks, employee (SICAM SL, Cajal PME, H&B), conference fees (Amgen, Asofarma, Astelas, Astra-Zeneca, Bayer, Boehringer Ingelheim, Faes, Janssen, Lilly, Msd, Novartis, Novo Nordisk, Viatrix), travel grants (Amgen, Lilly, Novonordisk, UCB), research fellowship (Amgen, Astra-Zeneca, Boehringer Ingelheim, Faes, Janssen, Lilly, Msd, Novo Nordisk Pfizer & Sanofi) and advisory boards (Amgen, Astrazeneca, Faes, Fresenius, Italfármaco, Janssen, Lilly, Msd, Mundipharma, Novo Nordisk, Shire & Ucb). Jorge Malouf Sierra: conference fees (Theramex, Amgen, Anghelini), travel grants (Lilly) and advisory boards (Amgen, UCB). Guillermo Martínez Díaz-Guerra: conference fees (Lilly, Amgen, Ucb, Angelini Pharma, Italfarmaco, Kyowa Kirin), travel grants (Lilly, Amgen, UCB), research fellowship (Amgen) and advisory boards (Lilly, Amgen, Ucb, Alexion, Shire, Kyowa Kirin). Ana Monegal Brancos: travel grants (Amgen, Lilly). Manuel Muñoz Torres: conference fees (Amgen, Ucb, Grünenthal Pharma, Stada, Meiji, Gedeon Richter, Ferrer) and advisory boards (Amgen, UCB, Meiji). Manuel Naves Díaz: conference fees (Grünenthal, Gedeon Richter) and travel grants (Amgen, Ucb). Xavier Nogués: conference fees (Ucb, Amgen, Lilly, Faes, Italfarmaco), travel grants (Amgen) and advisory boards (UCB, Amgen). Joan M Nolla: conference fees (Amgen, Lilly) and travel grants (Amgen, Lilly). José Luis Pérez-Castrillón: conference fees (Msd, Lilly, Amgen, Ucb, Gedeon-Ritcher, Gruggental), travel grants (Gedeon-Ritcher, Msd, Amgen, Italfarmaco), research fellowship (Pfizer) and advisory boards (Faes). Pilar Peris Bernal: conference fees (Amgen, UCB, Lilly, Kyowa Kirin). José Manuel Quesada Gómez: conference fees (Amgen, Faes, Ferrer, Gebro Pahrma, Grünental, Procare Health Iberia, S. L., Theramex), travel grants (Amgen, Faes), research fellowship (Faes) and advisory boards (Amgen, Shire). José A. Riancho: conference fees (Amgen, Ucb, Lilly, Merck), travel grants (Amgen, UCB, Lilly, Merck, Takeda) and becas de investigación (Alexion, Kyowa-Kirin). Minerva Rodríguez García: conference fees (Amgen, Kiowa Kyrin) and travel grants (Rubió, Amgen, Vifor). Carmen Valero Díaz de Lamadrid: conference fees (Amgen).
Please cite this article as: Riancho JA, Peris P, González-Macías J, Pérez-Castrillón JL, en nombre de la Comisión de Redacción de las Guías de Osteoporosis de la SEIOMM. Resumen ejecutivo de las guías de práctica clínica en la osteoporosis posmenopáusica, glucocorticoidea y del varón (actualización 2022). Sociedad Española de Investigación Ósea y del Metabolismo Mineral (SEIOMM). Revista Clínica Española. 2022;222:432–439.
The list with the names of the Editorial Committee is in Appendix A.
This document is published simultaneously in the journals Revista de Osteoporosis y Metabolismo Mineral (https://doi.org/10.4321/S1889-836X2022000100002) and Revista Clínica Española (https://doi.org/10.1016/j.rce.2021.12.007), with the consent of the authors and publishers.