To determine which patients within the high-risk group are most likely to have insufficient post-vaccination immunity.
MethodsDetermination of IgG titers against SARS-CoV-2 after the booster dose. Vaccine response was categorized as negative (IgG titers < 34 BAU/ml), indeterminate (titers 34–259 BAU/ml) or positive (≥260 BAU/ml).
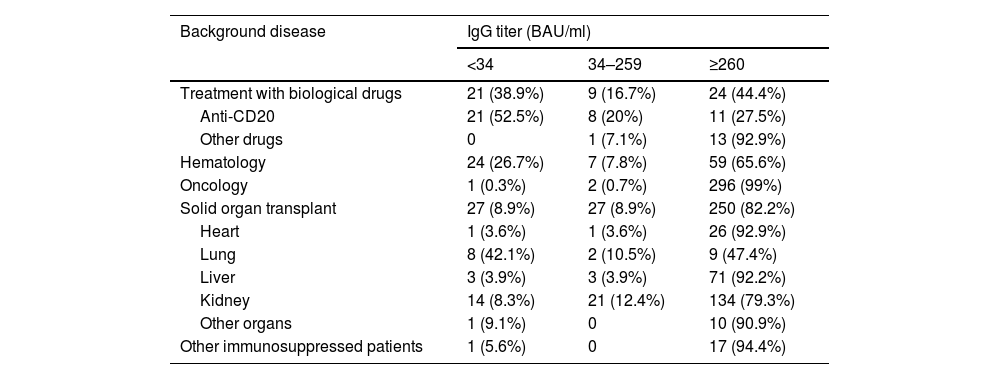
Results765 patients were included (31.25% of those vaccinated). 54 (7.1%) on treatment with biologics, 90 (11.8%) with hematologic disease, 299 (39.1%) with oncologic pathology, 304 (39.7%) with solid organ transplant and 18 (2.4%) with immunosuppression for other reasons. 74 patients (9.7%) had negative serology and 45 (5.9%) had indeterminate titers. By diagnostic group, the patients with the highest proportion of negative or indeterminate serology were patients with biologic treatment (55.6%, mainly at expense of antiCD20), hematologic (35.4%) and transplant patients (17.8%, mainly lung and kidney). Oncology and other immunosuppressed patients had a favorable response to vaccination.
ConclusionPatients treated with antiCD20 drugs, hematologic patients and transplanted patients (mainly lung and kidney) have a higher risk of not achieving post-vaccination immunity. It is essential to identify them in order to individualize and optimize their management.
Identificación dentro del grupo de pacientes de alto riesgo a aquellos que presentan más posibilidad de presentar inmunidad postvacunal insuficiente.
MétodosDeterminación de títulos de IgG frente a SARS-CoV-2 después de la dosis de recuerdo. Se clasificó la respuesta vacunal como negativa (títulos IgG < 34 BAU/ml), indeterminada (títulos 34–259 BAU/ml) o positiva (≥260 BAU/ml).
ResultadosSe incluyeron 765 pacientes (31,25% de los vacunados). 54 (7,1%) en tratamiento con fármacos biológicos, 90 (11,8%) con enfermedad hematológica, 299 (39,1%) con patología oncológica, 304 (39,7%) con trasplante de órgano sólido y 18 (2.4%) con inmunosupresión por otros motivos. 74 pacientes (9,7%) tuvieron una serología negativa y 45 (5,9%) obtuvieron títulos indeterminados. Por grupo diagnóstico los pacientes con mayor porcentaje de serología negativa o indeterminada fueron pacientes bajo tratamiento con fármacos biológicos (55,6%, fundamentalmente a expensas de antiCD20), hematológicos (35,4%) y los trasplantados (17,8%, principalmente pulmón y riñón). Los pacientes oncológicos y otros pacientes inmunosuprimidos tuvieron buena respuesta vacunal.
ConclusiónLos pacientes tratados con fármacos antiCD20, los hematológicos y los trasplantados (fundamentalmente de pulmón y riñón) presentaron mayor riesgo de no desarrollar inmunidad postvacunal. Es fundamental su identificación de cara a individualizar y mejorar su manejo.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<