To describe the predictors of mortality in hospitalized patients with severe acute respiratory syndrome (SARS) due to COVID-19 presenting with silent hypoxemia.
Material and methodsRetrospective cohort study of hospitalized patients with SARS due to COVID-19 and silent hypoxemia at admission, in Brazil, from January to June 2021. The primary outcome of interest was in-hospital death. Multivariable logistic regression analysis was performed.
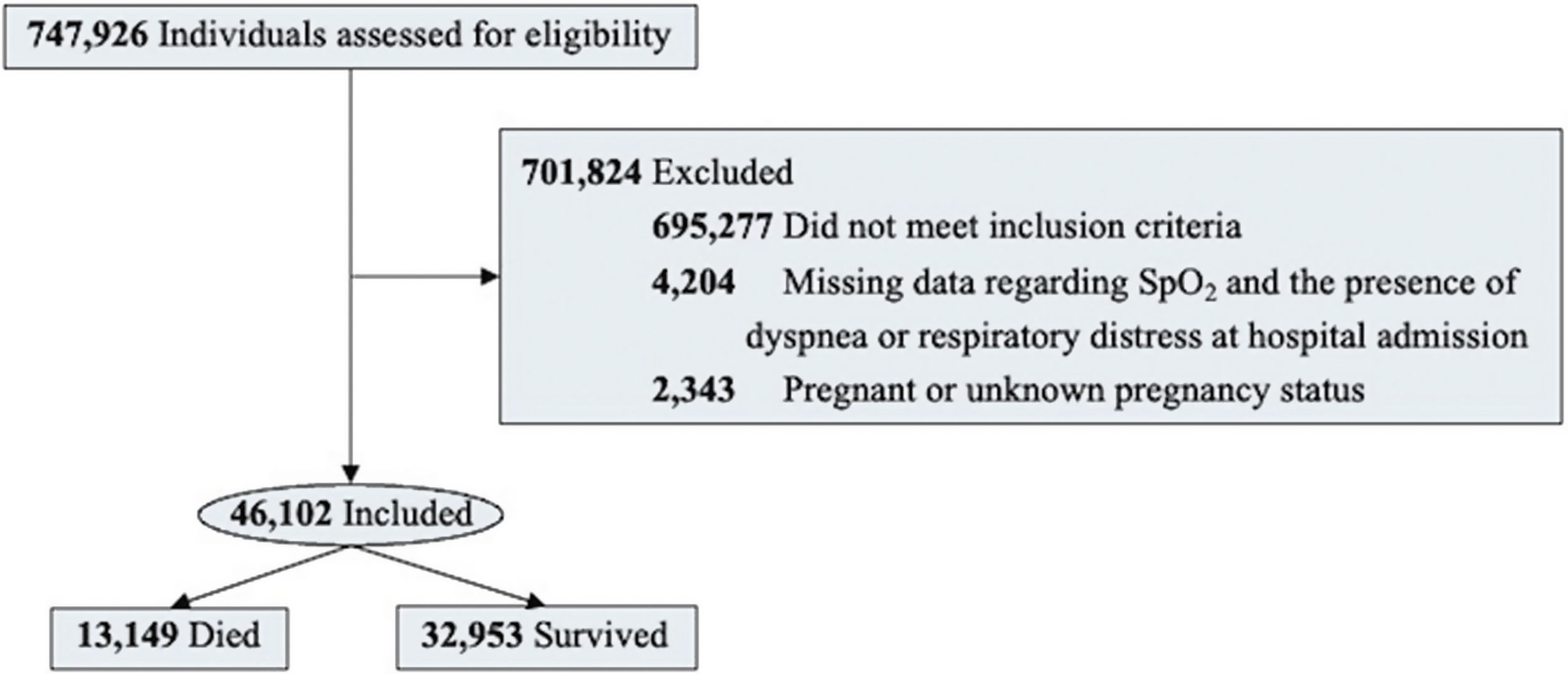
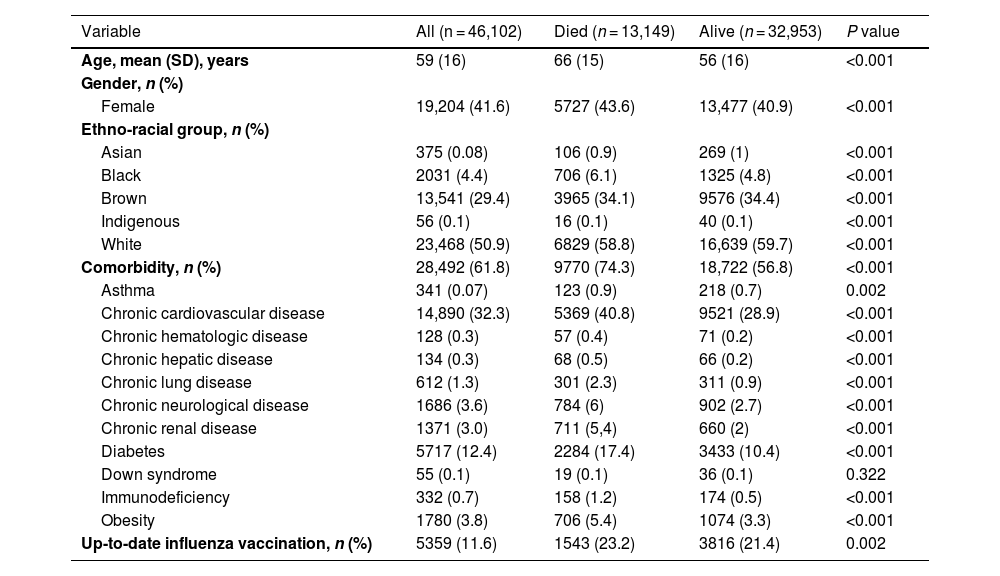
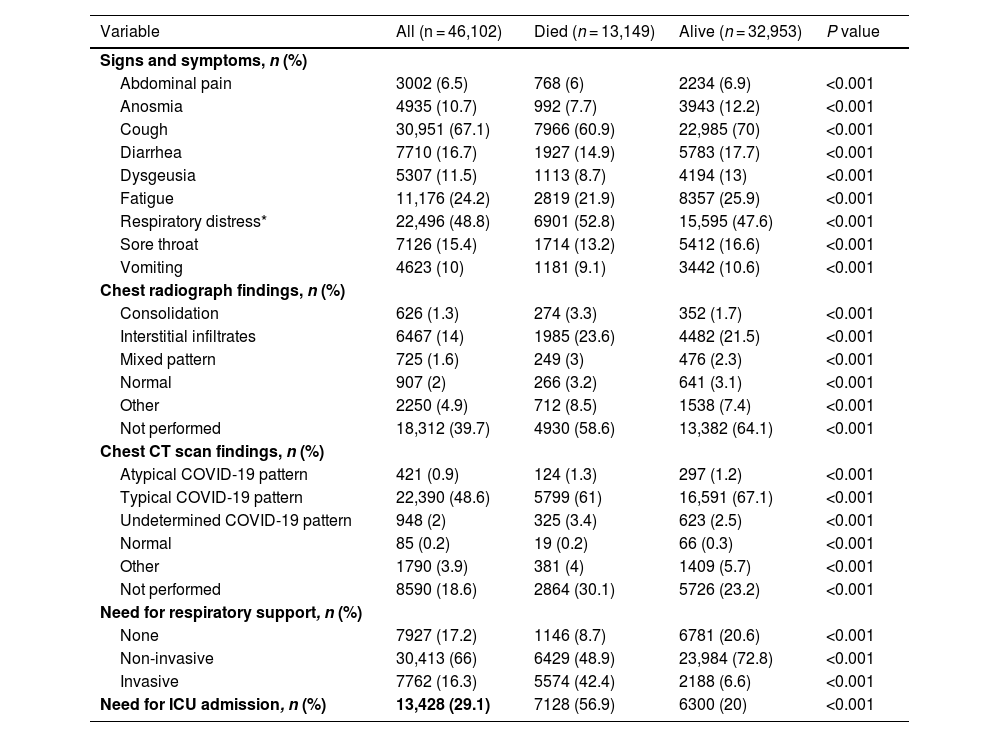
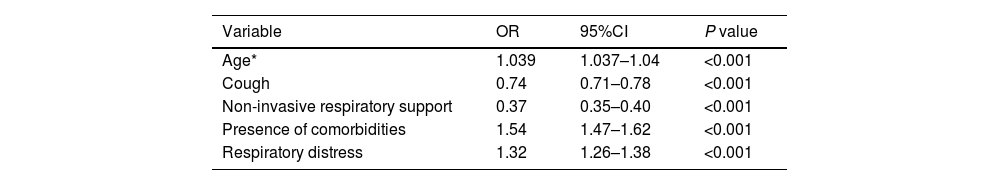
ResultsOf 46,102 patients, the mean age was 59 ± 16 years, and 41.6% were female. During hospitalization, 13,149 patients died. Compared to survivors, non-survivors were older (mean age, 66 vs. 56 years; P < 0.001), less frequently female (43.6% vs. 40.9%; P < 0.001), and more likely to have comorbidities (74.3% vs. 56.8%; P < 0.001). Non-survivors had higher needs for invasive mechanical ventilation (42.4% vs. 6.6%; P < 0.001) and intensive care unit admission (56.9% vs. 20%; P < 0.001) compared to survivors. In the multivariable regression analysis, advanced age (OR 1.04; 95%CI 1.037–1.04), presence of comorbidities (OR 1.54; 95%CI 1.47–1.62), cough (OR 0.74; 95%CI 0.71–0.79), respiratory distress (OR 1.32; 95%CI 1.26–1.38), and need for non-invasive respiratory support (OR 0.37; 95%CI 0.35–0.40) remained independently associated with death.
ConclusionsAdvanced age, presence of comorbidities, and respiratory distress were independent risk factors for mortality, while cough and requirement for non-invasive respiratory support were independent protective factors against mortality in hospitalized patients due to SARS due to COVID-19 with silent hypoxemia at presentation.
Describir los predictores de mortalidad en pacientes hospitalizados con síndrome respiratorio agudo severo (SARS) debido a COVID-19 que presentan hipoxemia silente.
Material y métodosEstudio de cohorte retrospectivo de pacientes hospitalizados con SARS debido a COVID-19 y hipoxemia silente al ingreso, en Brasil, de enero a junio de 2021. El resultado principal de interés fue la muerte intrahospitalaria. Se realizó un análisis de regresión logística multivariable.
ResultadosDe 46,102 pacientes, la edad media fue de 59 ± 16 años y el 41.6% eran mujeres. Durante la hospitalización, fallecieron 13,149 pacientes. En comparación con los sobrevivientes, los no sobrevivientes eran de mayor edad (edad media, 66 vs. 56 años; P < 0.001), menos frecuentemente mujeres (43.6% hombres vs. 40.9%; P < 0.001) y más propensos a tener comorbilidades (74.3% vs. 56.8%; P < 0.001). Los no sobrevivientes tuvieron mayores necesidades de ventilación mecánica invasiva (42.4% vs. 6.6%; P < 0.001) y admisión a la unidad de cuidados intensivos (56.9% vs. 20%; P < 0.001) en comparación con los sobrevivientes. En el análisis de regresión multivariable, la edad avanzada (OR 1.04; IC del 95% 1.037–1.04), la presencia de comorbilidades (OR 1.54; IC del 95% 1.47–1.62), la tos (OR 0.74; IC del 95% 0.71–0.79), la dificultad respiratoria (OR 1.32; IC del 95% 1.26–1.38) y la necesidad de soporte respiratorio no invasivo (OR 0.37; IC del 95% 0.35–0.40) permanecieron independientemente asociadas con la muerte.
ConclusionesLa edad avanzada, la presencia de comorbilidades y la dificultad respiratoria fueron factores de riesgo independientes para la mortalidad, mientras que la tos y la necesidad de soporte respiratorio no invasivo fueron factores protectores independientes contra la mortalidad en pacientes hospitalizados debido a SARS debido a COVID-19 con hipoxemia silente en la presentación.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<