N-Acetylcysteine has been proposed for the treatment of COVID-19 thanks to its mucolytic, antioxidant and anti-inflammatory effects. Our aim is to evaluate its effect on patients admitted with COVID-19 in mortality terms.
Material and methodsRetrospective single-center cohort study. All patients admitted to our hospital for COVID-19 from March to April 2020 have been considered.
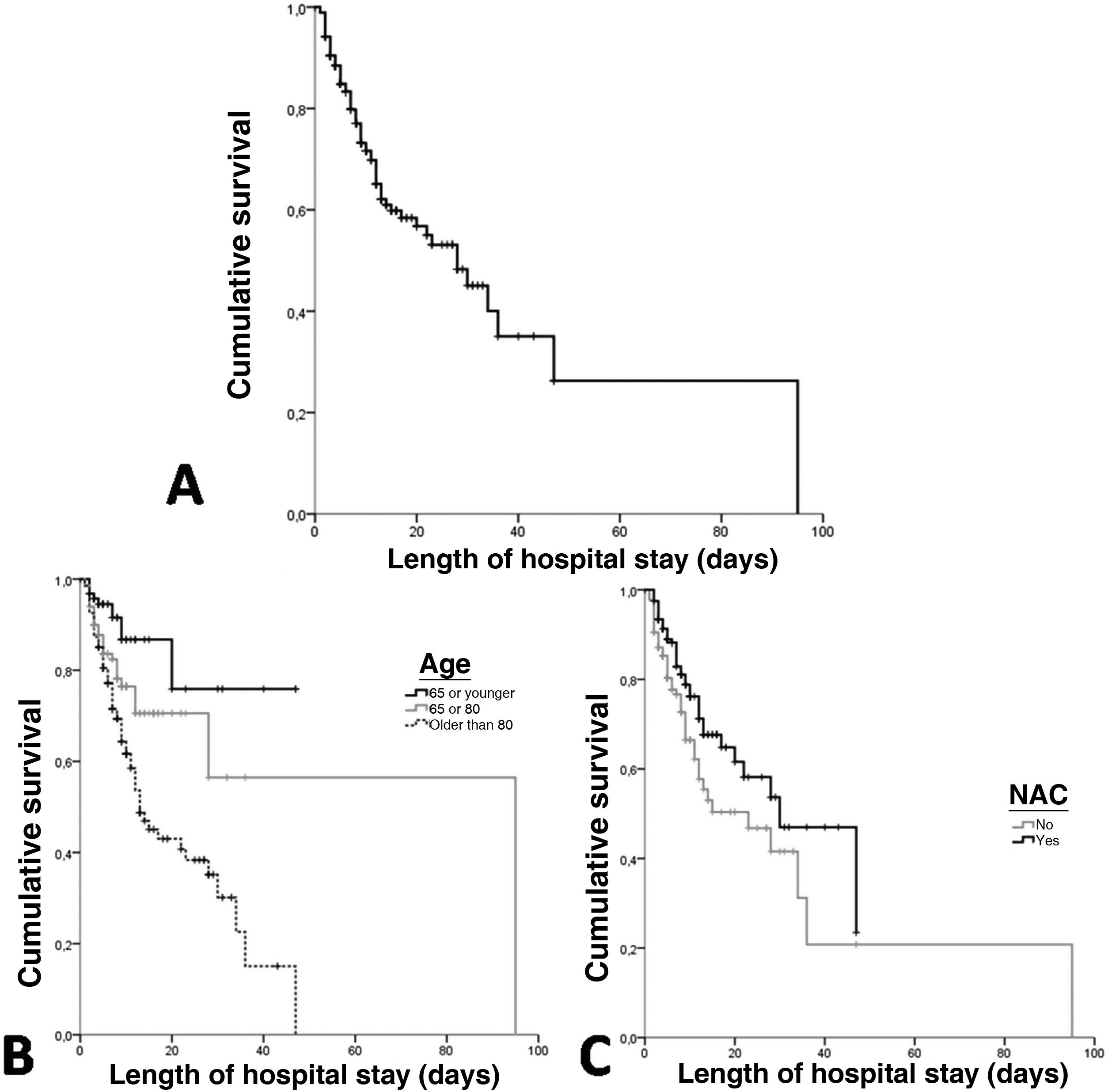
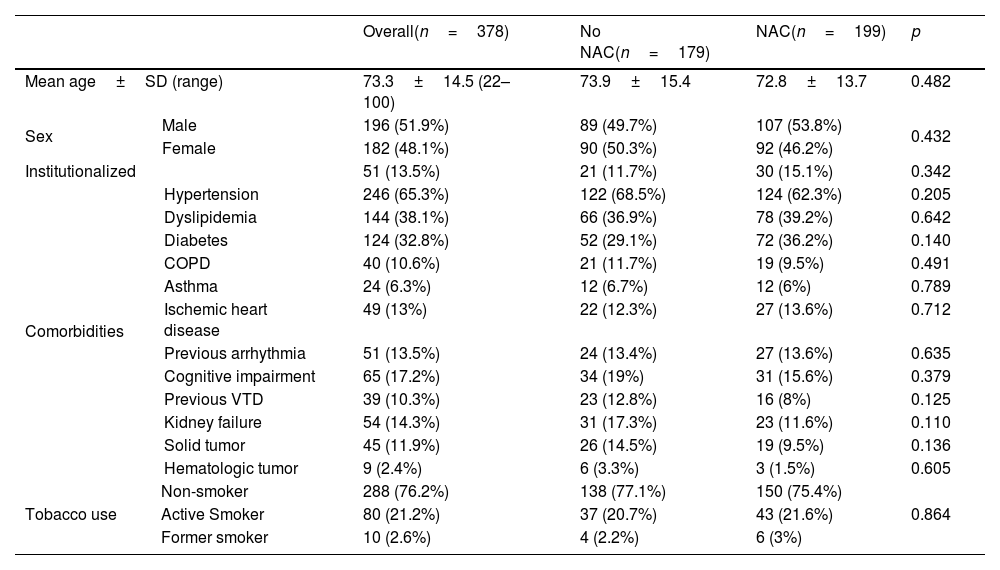
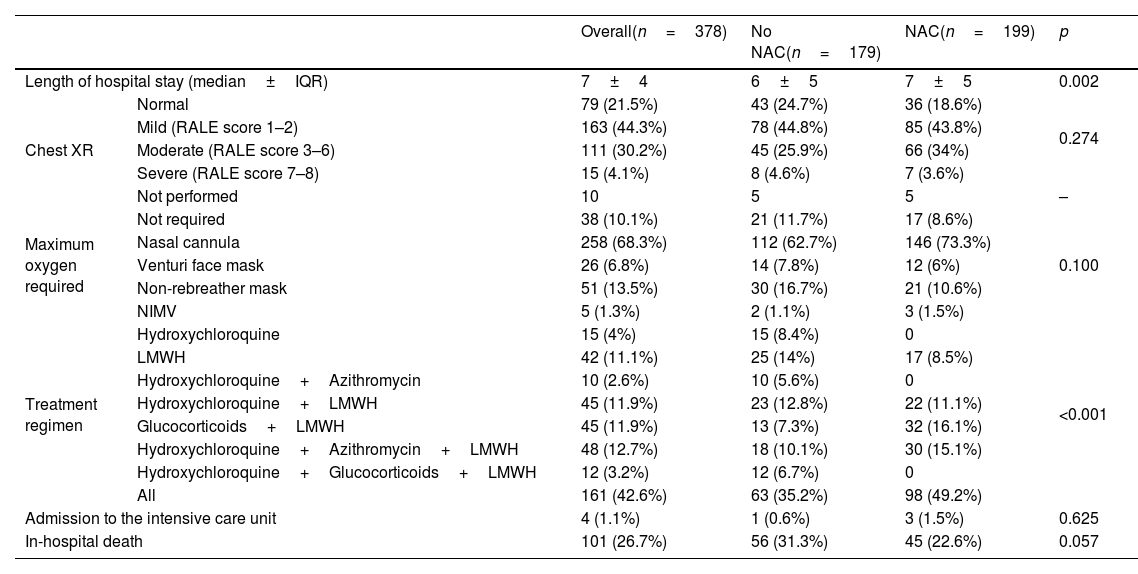
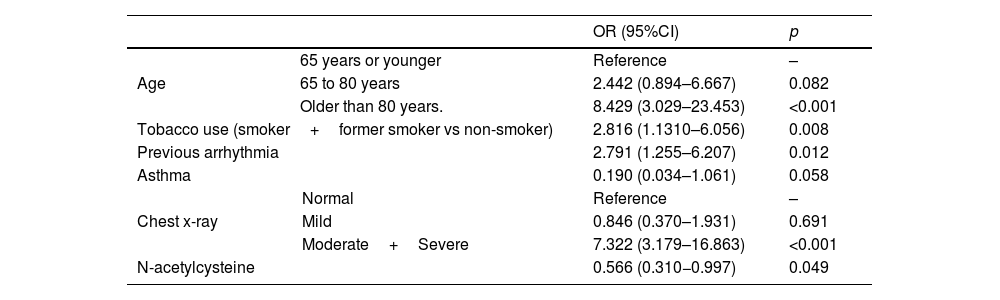
ResultsA total of 378 patients were included, being 196 (51.9%) men, with an average age of 73.3±14.5 years. 52.6% (199) received treatment with N-Acetylcysteine. More than 70% presented coughs, fever, and/or dyspnea. The global hospital mortality was 26.7%. A multivariate analysis through logistic regression identified the age of patients [older than 80; OR: 8.4 (CI95%:3−23.4)], a moderate or severe radiologic affectation measured by the RALE score [OR:7.3 (CI95%:3.2–16.9)], the tobacco consumption [OR:2.8 (CI95%:1.3–6.1)] and previous arrhythmia [OR 2.8 (CI95%: 1.3–6.2)] as risk factor that were independently associated with mortality during the admission. The treatment with N-Acetylcysteine was identified as a protective factor [OR: 0.57 (CI95%: 0.31−0.99)]. Asthma also seems to have a certain protective factor although it was not statistically significant in our study [OR: 0.19 (CI95%: 0.03–1.06)].
ConclusionsPatients with COVID-19 treated with N-acetylcysteine have presented a lower mortality and a better evolution in this study. Future prospective studies or randomized clinical trials must confirm the impact of N-Acetylcysteine on COVID-19 patients.
La N-Acetilcisteína se ha propuesto para el tratamiento de COVID-19 gracias a sus efectos mucolítico, antioxidante y antiinflamatorio. El presente estudio tiene como objetivo evaluar su efecto en pacientes ingresados con COVID-19, en términos de mortalidad.
Material y métodosEstudio de cohorte retrospectivo unicéntrico. Se incluyeron todos los pacientes ingresados por COVD-19 entre marzo y abril de 2020 en nuestro hospital.
ResultadosUn total de 378 pacientes fueron incluidos, de ellos 196 (51,9%) fueron hombres, la edad media fue de 73,3±14,5 años. 199 (52,6%) pacientes recibieron tratamiento con N-Acetilcisteína. Más del 70% tuvieron tos, fiebre y/o disnea. La mortalidad hospitalaria global fue del 26,7%. Un análisis multivariante mediante regresión logística identificó la edad de los pacientes [mayores de 80 años; OR: 8,4 (IC95%: 3–23,4)], una afectación radiológica moderada o grave medida por la escala RALE [OR: 7,3 (IC95%: 3,2–16,9)], el consumo de tabaco [OR: 2,8 (IC95%: 1,3–6,1)] y arritmia previa [OR: 2,8 (IC95%: 1,3–6,2)] como factores de riego que se asociaron independientemente con la mortalidad durante el ingreso. El tratamiento con N-acetilcisteína fue identificado como factor protector [(OR: 0,57 (IC95%: 0,31–0,99)]. El asma podría representar asimismo un factor protector de mortalidad, aunque en el presente estudio no alcanza significación estadística [(OR: 0,19 (IC95%: 0,03–1,06)].
ConclusionesLos pacientes con COVID-19 tratados con N-Acetilcisteína presentaron una menor mortalidad y mejor evolución en nuestro estudio. Futuros estudios prospectivos o ensayos clínicos aleatorizados deben confirmar el papel de la N-Acetilcisteína en pacientes con COVID-19.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<