The population with type 2 DM (DM2) is highly heterogeneous, representing an important challenge for healthcare professionals. The therapeutic choice should be individualized, considering the functional status, frailty, the occurrence of comorbidities, and the preferences of patients and their caregivers. New evidence on the cardiovascular and renal protection of specific therapeutic groups and on the usefulness of new technologies for DM2 management, among other aspects, warrant an update of the consensus document on the DM2 in the elderly that was published in 2018.
La heterogeneidad de la población de edad avanzada con DM tipo 2 (DM2) supone un reto importante para los profesionales de la salud. La elección del régimen terapéutico debe ser individualizada, considerando el estado funcional, la fragilidad y las comorbilidades, así como las preferencias del paciente y sus cuidadores. La nueva evidencia sobre la protección cardiovascular y renal de determinados grupos terapéuticos, así como la utilidad de nuevas tecnologías en el manejo de la DM2, entre otros aspectos, hace necesaria una actualización del documento de consenso sobre la DM2 en el paciente anciano que se publicó en 2018.
The older adult population with type 2 diabetes mellitus (DM2) is highly heterogeneous and requires personalized treatment goals and regimens.1 The choice of treatment must be individualized, seeking to maintain or improve quality of life and taking into account the balance between the benefits and possible risks of treatments.
It is essential to have updated information based on scientific evidence for this population. In 2013, a consensus article from various Spanish scientific societies was published on DM2 in older adults; its update was published in 2018.2 At present, new evidence has become available on the cardiovascular and renal protection of glucagon-like peptide-1 receptor agonists (GLP-1ra) and sodium-glucose cotransporter 2 inhibitors (SGLT-2i) as well as on the use of telemedicine in this population, among other aspects. This has made it advisable to update the consensus document published in 2018.
MethodsThe creation of the consensus document consisted of three phases. A scientific committee comprising four investigators reviewed the available updated literature of studies on the treatment of DM2 in the older adult and frail population. With this, a draft of the update of the 2018 consensus was created.2
In a second phase, the document was critically reviewed by eight investigators who represented the following scientific societies: the Spanish Diabetes Society (SED), the Spanish Society of Endocrinology and Nutrition (SEEN), the Spanish Society of Family and Community Medicine (semFYC), the Spanish Society of General and Family Physicians (SEMG), the Spanish Society of Primary Care Physicians (SEMERGEN), and the Spanish Society of Internal Medicine (SEMI).
The third phase consisted of another literature review followed by a discussion by the scientific committee which culminated in the 2022 Treatment of Type 2 Diabetes Mellitus in Older Adult Patients Consensus Document (Banexo Appendix, supplementary tables).
ResultsGiven the scarcity of existing research in this population, the consensus document responds to the need to approach the management of older adult or frail patients with DM2 in an individualized manner. To do so, various thematic areas necessary for the comprehensive treatment of this population are addressed.
First, the target population of the consensus document and the necessary screening tests for their assessment are defined. The main comorbidities in this population are also specified, highlighting the importance of preventing hypoglycemic episodes. The consensus document defines the main blood glucose targets based on the older adult or frail patient’s clinical condition.3
Given its relevance, the consensus document focuses on the non-pharmacological and pharmacological approach to the older adult population with DM2, describing the main drug classes (metformin, sulfonylureas, meglitinides, alpha-glucosidase inhibitors, thiazolidinediones, dipeptidyl peptidase-4 inhibitors (DPP-4i),4 GLP-1ra,5–7 SGLT-2i,8–10 and insulin11) and including recommendations and the main outcomes of studies conducted in this population. The drug treatment section places special emphasis on the cardiovascular and renal protection outcomes of the GLP-1ra and SGLT-2i drug classes that have spurred the update of this document. A section is also dedicated to describing combined therapies, indicating the preferred treatment combinations for each clinical situation.
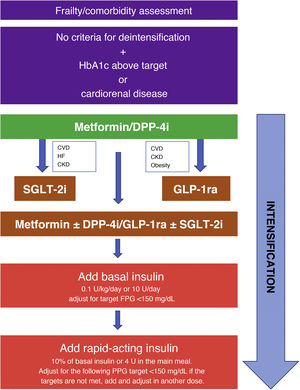
Of particular relevance is the section dedicated to intensification and deintensification algorithms, in which strategies are provided for starting, intensifying, and deintensifying treatment tailored to various clinical situations. They are shown graphically in Fig. 1.
Strategy for starting and intensifying hypoglycemic treatment and therapy with full insulin regimens. GLP-1ra: GLP-1 receptor agonists; CVD: cardiovascular disease; CKD: chronic kidney disease; FPG: fasting plasma glucose; PPG: preprandial plasma glucose; HF: heart failure; DPP-4i: DPP-4 inhibitors; SGLT-2i: SGLT-2 inhibitors.
Lastly, the results describe advances in telemedicine in DM2, highlighting the main applications in the older adult population.12–14
DiscussionThe consensus document is intended to be a useful guide for healthcare professionals in various disciplines involved in caring for older adult or frail patients. The limitations of this consensus document include the lack of clinical research in this population and the heterogeneity of the target population, which prevent its universal validation. For all of the above, it is essential that it be applied in an individualized manner and under the physician’s clinical judgment.
FundingAbbott Diabetes España made an unconditional financial contribution for assistance in medical writing. The conception and interpretation of the existing evidence was conducted by the authors.
Conflicts of interestFG-P (Fernando Gómez-Peralta) has served as an advisor for Abbott Diabetes, AstraZeneca, Novartis, Novo Nordisk, and Sanofi; has been an investigator in clinical trials for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Sanofi; and has served as a speaker for Abbott Diabetes, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Novartis, Novo Nordisk, and Sanofi.
FJC-S (Francisco Javier Carrasco-Sánchez) has performed advising tasks for Boehringer-Lilly, Novo Nordisk, Sanofi, AstraZeneca, MSD, Janssen; has done remunerated work for AstraZeneca, Boehringer-Lilly, Novartis, Novo-Nordisk, and Sanofi; and has done research studies for Boehringer-Lilly, Novo Nordisk, Sanofi, and Janssen.
AP (Antonio Pérez) has done consulting activities and has received support for research, fees for conferences, or the reimbursement of travel expenses from Sanofi, Almirall, Novo Nordisk, Lilly, MSD, Boehringer Ingelheim, Esteve, Gilead, Novartis, Abbott, Amgen, Menarini, and AstraZeneca.
JE (Javier Escalada) has served as an advisor for Abbott Diabetes, AstraZeneca, Eli Lilly, MSD, Novo Nordisk, and Sanofi; has been an investigator in clinical trials for Eli Lilly and Novo Nordisk; and has served as a speaker for AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk, and Sanofi.
FA-G (Fernando Álvarez-Guisasola) has served as an advisor for Sanofi, Novo Nordisk, Mundipharma, and Janssen; as a speaker for AstraZeneca, Boehringer-Ingelheim, Lilly, MSD, Novo Nordisk, and Sanofi; and has been an investigator in clinical trials for Novo Nordisk and MSD.
CM-F-S (Carlos Miranda-Fernández-Santos) has performed advising tasks for Boehringer-Lilly, Novo Nordisk, Sanofi, AstraZeneca, MSD, Janssen, and Mundipharma.
JJM-B (José Javier Mediavilla-Bravo) has served as an advisor for Lilly, MSD, Mundipharma, Novo Nordisk, and Sanofi and has received fees for conferences from AstraZeneca, Boehringer-Ingelheim, Lilly, MSD, Novo Nordisk, and Sanofi.
RG-H (Ricardo Gomez-Huelgas) has performed advising tasks for Boehringer-Lilly, Novo Nordisk, Sanofi, AstraZeneca, MSD, Janssen; has done paid work for AstraZeneca, Boehringer-Lilly, Novartis, Novo Nordisk, and Sanofi; and has done research studies for Boehringer-Lilly, Novo Nordisk, Sanofi, and Janssen.
We would like to thank Carla Granados of Trialance SCCL for providing assistance in the writing and layout.
Please cite this article as: Gómez-Peralta F, Carrasco-Sánchez FJ, Pérez A, Escalada J, Álvarez-Guisasola F, Miranda-Fernández-Santos C, et al. Resumen ejecutivo sobre el tratamiento de la diabetes mellitus tipo 2 en personas de edad avanzada o frágiles. Actualización 2022 del documento de consenso 2018 «Tratamiento de la diabetes mellitus tipo 2 en el paciente anciano». Rev Clin Esp. 2022;222:496–499.
The complete consensus document is available as Appendix A in Supplementary Material.