The heterogeneity of patients with heart failure and preserved ejection fraction (HFpEF) is high, thus this entity tends to be grouped into phenotypes to act with precision. Within these groups, patients with type 2 diabetes mellitus (DM2) hold this heterogeneity. Our aim is to describe subgroups of patients with HFpEF and DM2 based on other comorbidities.
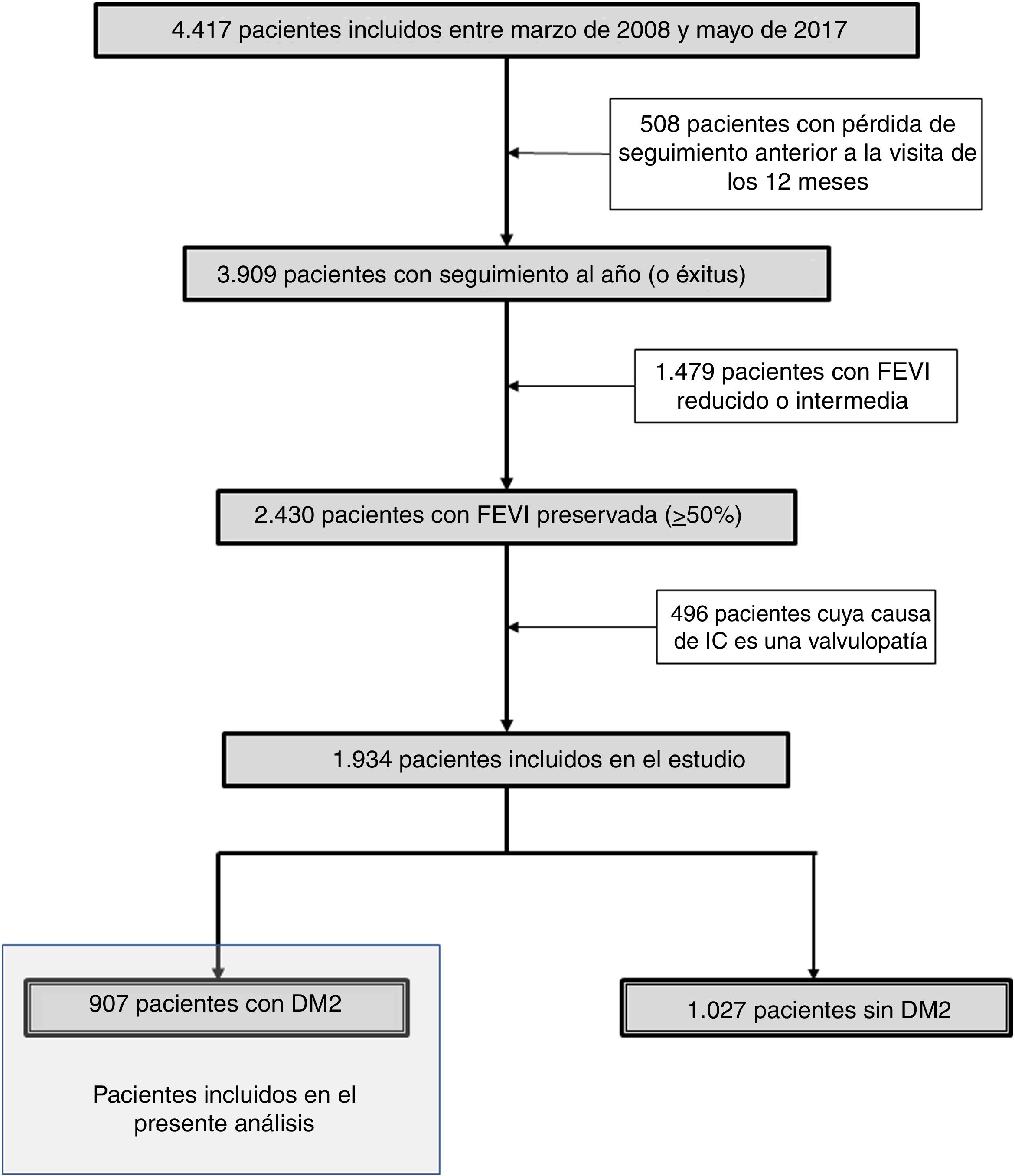
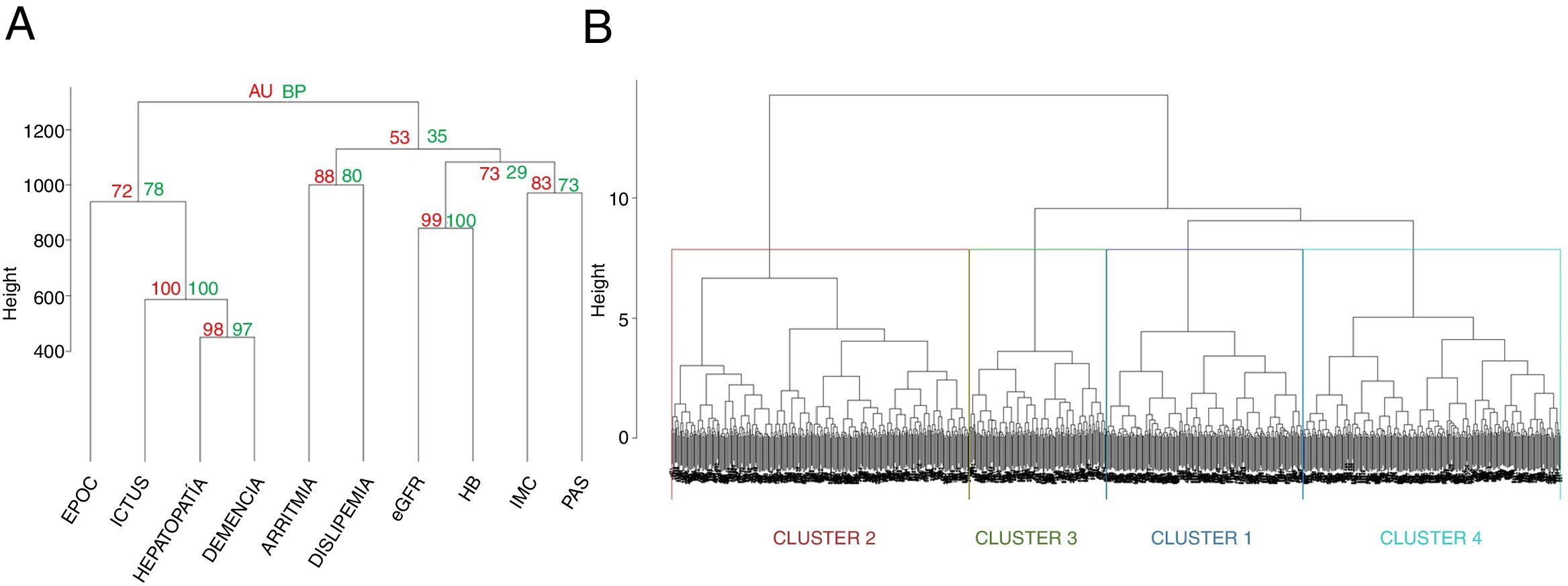
Material and methodsPatients were recruited from the national registry of heart failure (RCIA). Patients with ejection fraction greater than or equal to 50% without valvular disease and with DM2 were included. A hierarchical agglomerative analysis was performed with Ward's method including the following variables: dyslipidemia, liver disease, Chronic obstructive pulmonary disease (COPD), dementia, cerebrovascular disease, arrhythmia, systolic blood pressure, body mass index (BMI), estimation of glomerular filtration and hemoglobin.
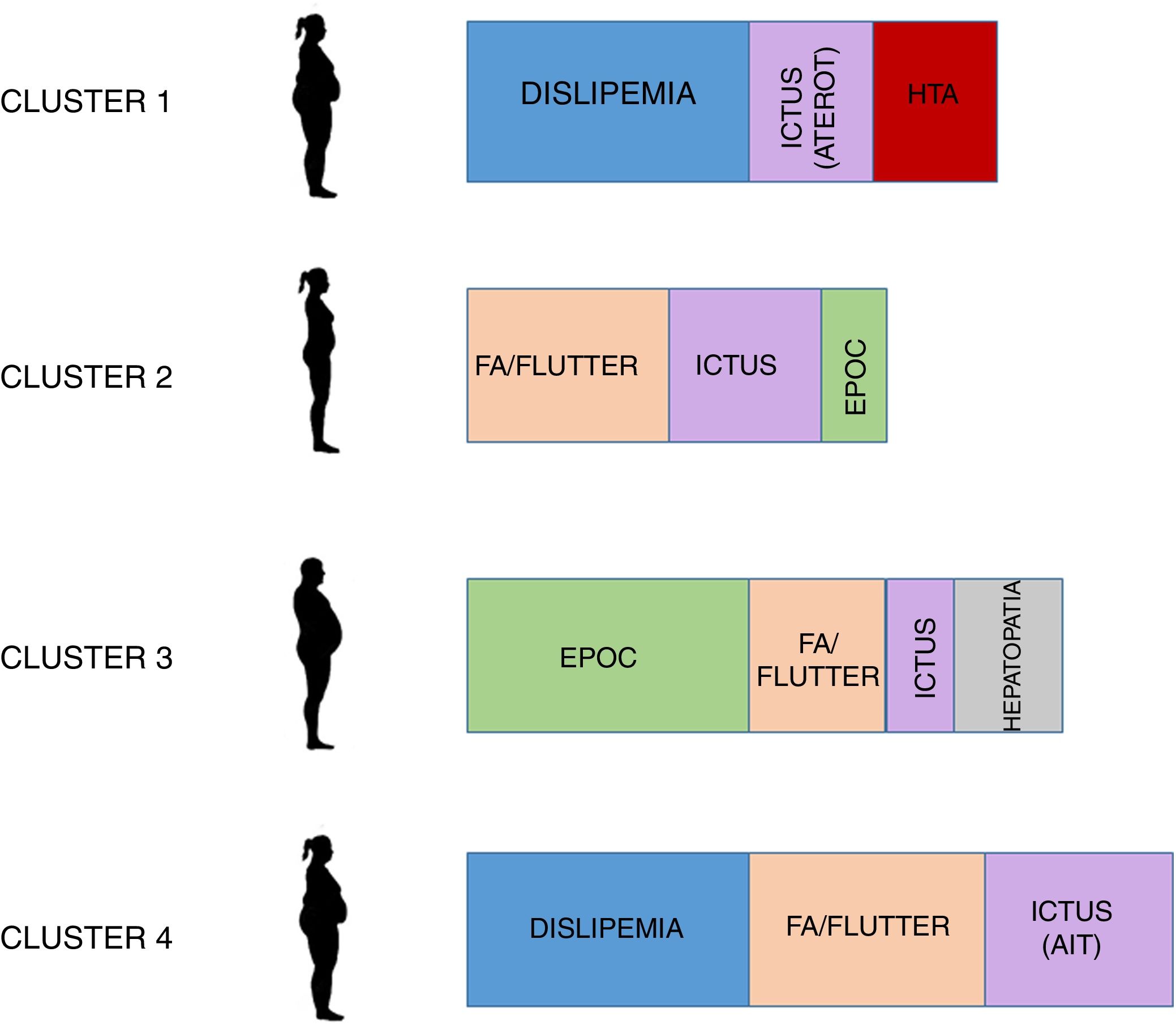
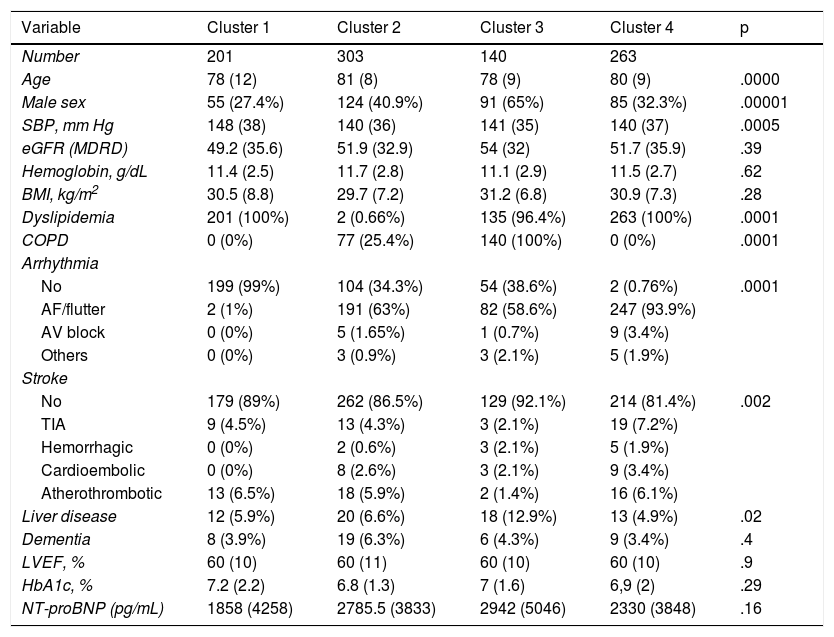
ResultsA total of 1934 patients with HFpEF were included, of which 907 (46.9%) had DM2 with a predominance of women (60.9%) and with a BMI of 31.1 (5.9) Kg/m2. Four groups were obtained, two with high vascular risk (one with arrhythmia and the other without it) with 263 patients the first and 201 the second. A third group had a predominance of COPD (140 patients) and a last group with 303 patients older but with less comorbidity.
ConclusionsIn our patients with HFpEF and DM2, obesity and female sex predominated. All four groups offered treatment chances to improve their prognosis not only based on the use of new antidiabetic drugs but also on other options that may be a starting point for further research
La heterogeneidad de los pacientes con insuficiencia cardíaca y fracción de eyección preservada (HFpEF) es elevada, por lo que se tiende a agrupar en fenotipos para intervenir con precisión. Dentro de estos, los pacientes con diabetes mellitus (DM) mantienen esta heterogeneidad. Nuestro objetivo es describir grupos de pacientes con ICFEP y DM basados en otras comorbilidades.
Material y métodosLos pacientes se reclutan desde el registro nacional de insuficiencia cardíaca (RICA). Se incluyen pacientes con fracción de eyección mayor o igual al 50% sin valvulopatía y con DM. Se realiza un análisis aglomerativo jerárquico con el método de Ward incluyendo las siguientes variables: dislipemia, hepatopatía, EPOC, demencia, enfermedad cerebrovascular, arritmia, presión arterial sistólica, índice de masa corporal (IMC), estimación del filtrado glomerular y hemoglobina.
ResultadosSe incluyen 1.934 pacientes con ICFEP, de los que 907 (46,9%) tenían DM, con predominio de mujeres (60,9%) y con un IMC de 31,1 (5,9) kg/m2. Se obtienen 4 grupos: dos con elevado riesgo vascular (uno con arritmia y otro no), con 263 pacientes el primero y 201 el segundo, otro con predominio de EPOC (140 pacientes) y un último grupo de 303 pacientes con más edad pero menos comorbilidad.
ConclusionesEn nuestros pacientes con ICFEP y DM predomina la obesidad y el sexo femenino. Los cuatro grupos ofrecen oportunidades de tratamiento para mejorar su pronóstico no solo basadas en la utilización de nuevos fármacos antidiabéticos sino por otras opciones que pueden suponer un punto de partida para nuevas investigaciones.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<