There are different second line glucose lowering drugs whose efficacy, safety and economic profile have not been established in our setting. We have analyzed the clinical (diabetic treatment adherence, metabolic control, hypoglycemia and macrovascular complications) and economic (resource use and costs) consequences of the combination of metformin with dipeptidyl peptidase inhibitors (DPPIV) in patients with type 2 diabetes.
Patients and methodsWe conducted a multicenter, observational and retrospective study. Patients ≥30 years treated with metformin who initiated a second antidiabetic treatment during 2008–2009 were enrolled in the study. Two groups of patients were established: metformin with DPPIV and metformin with other diabetic drugs. The main measurements were comorbidity, compliance/persistence, metabolic control (glycosylated hemoglobin <7%), complications (hypoglycemia, macrovascular) and total costs. Patients were followed-up for 2 years.
ResultsA total of 2067 patients were enrolled (mean age: 66.6 years, 53.1% male). Of these, 519 patients (25.1%) were analyzed in the metformin+DPPIV group and 1548 patients (74.9%) in the group metformin+other antidiabetic drug. The DPPIV group patients showed better compliance (70.3 vs. 59.6%), persistence (63.4 vs. 51.0%) and metabolic control (64.3 vs. 59.6%), respectively (P<.001) compared to the other group. They also showed a lower proportion of hypoglycemia (13.9 vs. 44.3%), cardiovascular events (3.7 vs. 7.6%) and total costs (2347 vs. € 2682), P<.05.
ConclusionsDespite the limitations of the study, patients treated with metformin associated to DPPIV were more likely to show increased adherence, metabolic control and lower rates of hypoglycemia than those treated with metformin associated to other antidiabetics.
Existen diversos tratamientos farmacológicos hipoglucemiantes de segunda línea cuya eficacia, seguridad y perfil económico no se ha precisado en nuestro medio. Hemos analizado las consecuencias clínicas (adherencia al tratamiento antidiabético, control metabólico, hipoglucemias y complicaciones macrovasculares) y económicas (uso de recursos y costes) de la combinación de metformina con inhibidores de la dipeptidilpeptidasa (IDPP4) en pacientes con diabetes tipo 2 en comparación con metformina y otros fármacos hipoglucemiantes.
Pacientes y métodosEstudio observacional multicéntrico de carácter retrospectivo. Se incluyeron pacientes de ≥30 años tratados con metformina que iniciaron un segundo tratamiento antidiabético durante los años 2008-2009. Se establecieron 2 grupos de pacientes: metformina con IDPP4 y metformina con otros fármacos antidiabéticos. Las principales medidas fueron: la comorbilidad, el cumplimiento/persistencia, el control metabólico (hemoglobina glicosilada<7%), complicaciones (hipoglucemias, macrovasculares) y costes totales. El seguimiento se realizó durante 2 años.
ResultadosSe reclutaron 2.067 pacientes (edad media: 66,6 años; 53,1% varones). En el grupo metformina+IDPP4 se analizaron 519 pacientes (25,1%) y en el grupo metformina+otros fármacos antidiabéticos: 1.548 pacientes (74,9%). Los enfermos tratados con IDPP4, en comparación con los que recibieron metformina asociada a otros antidiabéticos, mostraron un mejor cumplimiento (70,3 vs. 59,6%), persistencia (63,4 vs. 51,0%) y control metabólico (64,3 vs. 59,6%) (p<0,001). También presentaron una menor proporción de hipoglucemias (13,9 vs. 44,3%), eventos cardiovasculares (3,7 vs. 7,6%) y costes totales (2.347 vs. 2.682€) (p<0,05) durante los 2 años del estudio.
ConclusionesA pesar de las limitaciones del estudio, los pacientes en tratamiento con metformina asociada a IDPP4 mostraron un mayor cumplimiento terapéutico, control metabólico y menores tasas de hipoglucemias que los enfermos tratados con metformina asociada a otros antidiabéticos.
The safety, efficacy and economic profile of hypoglycemic drugs associated with metformin have not been specified for our community.
What this article provides?Patients with type 2 diabetes mellitus who were treated with metformin associated with IDPP4 showed greater treatment compliance, better metabolic control and lower rates of hypoglycemia than patients treated with metformin associated with other antidiabetic agents.
The Editors
Type 2 diabetes (DM) is a disease with one of greatest healthcare impacts, not only for its high prevalence but also for the acute and chronic complications it causes, for its high mortality rate and repercussions on the quality of life and for the high consumption of healthcare resources it entails.1–3 The prevalence of type 2 DM in Spain is approximately 8% in women and 12% in males and varies between 6% and 12%, reaching up to 20% in those older than 75 years of age.4,5
The objective of drug treatment in type 2 DM is to achieve proper metabolic control with maximum safety. Metformin is the drug recommended as the first therapeutic step, along with the adoption of hygienic and dietary measures.6,7 When the patient's glycemic control is not sufficient with monotherapy, the addition of a second drug is recommended.8,9 The most common acute complication in patients with diabetes is hypoglycemia, especially in those treated with insulin and/or sulfonylureas.6,9–11 In this respect, the new therapeutic class of dipeptidyl peptidase-4 inhibitors (DPP-4i) has the advantage, when compared with traditional secretagogues, of considerably reducing hypoglycemia, given that its stimulator mechanism for insulin secretion is glucose-dependent.7,12
Data on the clinical and economic consequences of double drug therapy with antidiabetic agents in a population in Spain are limited or nonexistent. We have described the clinical (treatment compliance, metabolic control, hypoglycemia and macrovascular complications) and economic (use of resources and their costs) consequences of combining metformin with dipeptidyl peptidase inhibitors in patients with type 2 diabetes.
Patients and methodsStudy design and populationWe conducted an observational, multicenter, longitudinal study based on a review of medical registries (computer databases) of patients treated with metformin and monitored in outpatient and hospital settings. The study population consisted of individuals attending 6 remodeled primary care centers managed by Badalona Serveis Assistencials SA. Information was obtained on the resources consumed by this reference population from 2 hospital centers: Municipal Hospital of Badalona and Hospital Germans Trías i Pujol (hospital admissions). The population assigned to the centers was mostly urban, with a low to medium socioeconomic level, predominantly industrial.
Inclusion and exclusion criteriaWe included all patients who started a second antidiabetic treatment between 1/January/2008 and 31/December/2009 and who had the following characteristics: (a) men and women aged ≥30 years; (b) diagnosis of type 2 DM for a minimum of 12 months before the start of the study; (c) those regularly following the protocol/guidelines of cardiovascular risk established in the centers; (d) those who were part of the long-term prescription program to obtain prescriptions (with a confirmed record of the daily dosage, the time interval and duration of each treatment administered); (e) on current therapy with metformin as the first therapeutic option (monotherapy); and (f) those who could participate in follow-up for a period of 2 years. We excluded those who were transferred to other municipalities, those who were displaced or outside the area and those who only visited specialists.
Measure of type 2 diabetes and complicationsThe diagnosis of type 2 DM was determined based on the international classification of primary care (ICPC-2), component 7 of diseases and health problems,13 and on the coding of hospital discharges and emergency department visits, according to the international classification of diseases (9th edition, clinical modification [ICD-9-CM]). We obtained information on microvascular complications: (a) diabetic retinopathy; (b) diabetic nephropathy; (c) diabetic neuropathy; and (d) diabetic vasculopathy. We also identified all cases of symptomatic hypoglycemia.
Macrovascular complications and cardiovascular diseases (CVD)These included (a) heart disease, such as cardiac ischemia, acute myocardial infarction and heart failure, according to the definition of the diagnostic criteria of the World Health Organization; (b) cerebrovascular disease, including stroke (ischemic or hemorrhagic; according to the American Heart Association)7 and transient ischemic attack; (c) peripheral arterial disease (all types); and (d) kidney disease (diabetic nephropathy and renal function impairment [serum creatinine: men>133; women>124mmol or glomerular filtration rate<60ml/min]). The cumulative incidence rate was defined as the proportion of healthy individuals who developed the complication (number of new cases).
Compliance, treatment persistence and metabolic controlWe obtained information on the following antidiabetic agents according to the Anatomical Therapeutic Chemical Classification System (ATC)14: (a) metformin (A10BA*); (b) insulin release stimulants: sulfonylureas (A10BB*) and glinides (A10BX*); (c) glitazones (A10BG*); (d) DPP-4i (A10BH*); and (e) insulin (all types) in monotherapy or in combination (A10BD*). Patients taking alpha-glucosidase inhibitors were not included due to the insufficient sample size. Compliance for the period was calculated using the ratio between the total number of tablets dispensed and those recommended or prescribed. Treatment persistence was defined as the time (in months) without withdrawing from the initial therapy or without changing to another medication for at least 30 days after the initial prescription. Metabolic control was established based on glycated hemoglobin levels (HbA1c) <7%.6
Demographic and comorbidity variablesAs a summary variable for overall comorbidity for each treated patient, we used (a) the Charlson comorbidity index15 as an approximation of patient severity and (b) the individual case-mix index, obtained based on the Adjusted Clinical Groups (ACG), which is a patient classification system by isoresource consumption.16 The applied ACG provides resource utilization bands (RUBs), whereby each patient is grouped, according to their overall morbidity, into 1 of 5 mutually exclusive categories (1: healthy or very low morbidity; 2: low morbidity; 3: moderate morbidity; 4: high morbidity; and 5: very high morbidity).
Use of resources and cost modelWe considered direct healthcare costs to be those related to the health care activities conducted by professionals. The nonhealthcare or indirect costs were those related to the loss of work productivity (number of people on disability and sick days). The various study concepts and their economic assessments are detailed in Table 1 (corresponding to 2011). The various fees were obtained from the centers’ accounting analysis, except for the medication and sick leave. The work disability days or loss of productivity were considered nonhealthcare costs (indirect costs). The cost was quantified according to the minimum interprofessional wage (source: National Statistics Institute of Spain).17
Economic assessment of unit costs and losses of work productivity.
| Healthcare and nonhealthcare resources | Unit costs (€) |
| Medical Visits | |
| Primary Care Medical Visit | 23.19 |
| Emergency Department Medical Visit | 117.53 |
| Hospitalization (1 day) | 320.90 |
| Specialized Care Medical Visit | 104.41 |
| Additional examinations | |
| Laboratory Tests | 22.30 |
| Conventional radiology | 18.50 |
| Diagnostic tests | 37.12 |
| Drug prescription | PVP+VAT |
| Indirect work productivity costs | |
| Cost per day NOT worked | 101.21 |
PVP+VAT: price of sale to the public with VAT.
Source of healthcare resources: center's own accounting analysis. Values expressed in euros (€).
Source: Spanish National Institute of Statistics. Costs belonging to 2011.
We performed a descriptive-univariate statistical analysis with values for the mean, standard/typical deviation (SD) and 95% confidence intervals (CI). We determined the normality of the distribution with the Kolmogorov–Smirnov test for quantitative variables. In the bivariate analysis, we used ANOVA, chi-squared, or Pearson's linear correlation and the nonparametric Mann–Whitney–Wilcoxon tests. We performed a logistic regression analysis to determine the comorbidities associated with the DPP-4i group and another to define the variables associated with CVD (presence/absence), with the enter procedure (statistic: Wald). Comparisons of the outpatient and hospital costs were performed according to the recommendations of Thompson and Barber18 using analysis of covariance (ANCOVA), with gender, age, RUBs, Charlson index and progression time of the diagnosis as covariates.
ResultsFrom a selection of 62,370 patients, 6620 patients were diagnosed with type 2 DM (prevalence: 10.6%; 95% CI: 10.4–10.8%). A total of 4553 patients were excluded from the study: 978 were not undergoing a drug treatment, 464 were being treated with other drug therapies, 241 discontinued the therapy, 1887 modified the therapy during follow-up, 655 were considered lost to follow-up and 328 were excluded for unknown or other causes. The percentage distribution of the excluded patients in the 2 study groups was similar.
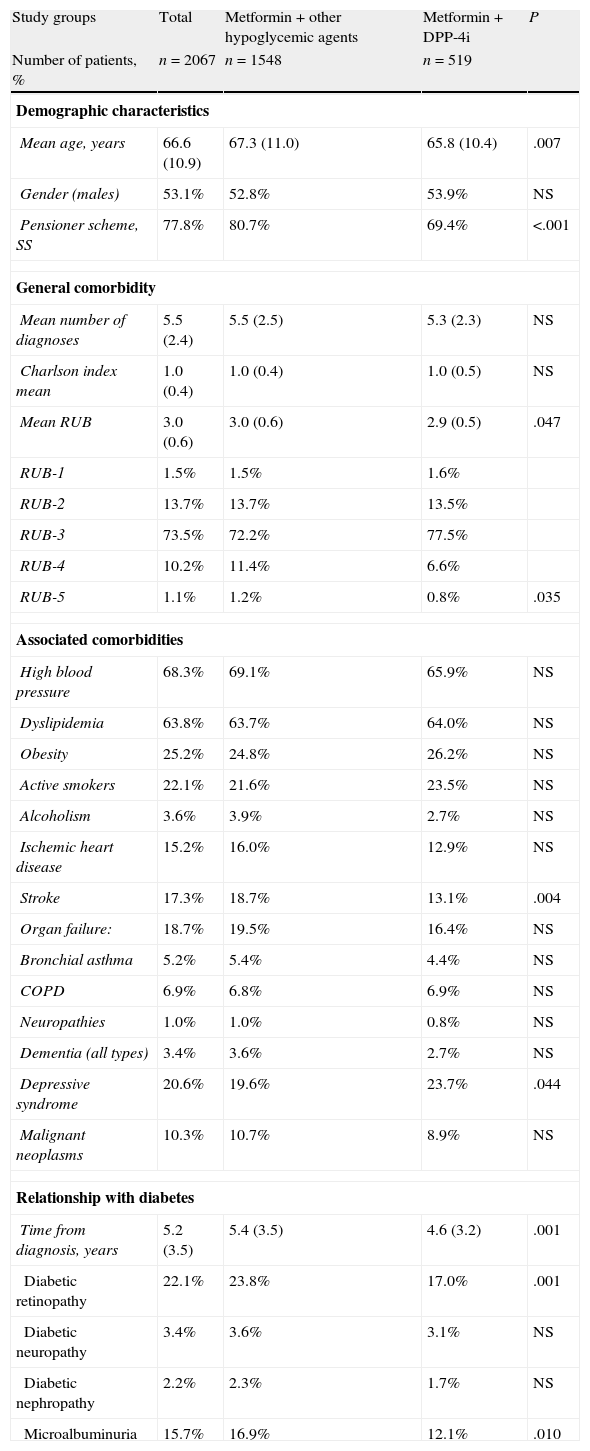
We selected 2067 patients on therapy with antidiabetic agents (double therapy). The mean age was 66.6 (SD: 10.9) years and 53.1% were male. Some 25.1% (n=519) were being treated with metformin and a DPP-4i, and 74.9% (n=1548) were being treated with metformin and other non-DPP-4i hypoglycemic drugs. The distribution of second-line drugs in this group was as follows: 42.9% (n=886) were treated with sulfonylureas, 14.0% (n=290) with glitazones and 18.0% (n=372) with insulin (Table 2). The patients on therapy with DPP-4i had a lower mean age than the reference group (65.8 vs. 67.3 years; P=.007) and had similar overall comorbidity (5.3 vs. 5.5 diagnoses, respectively). The patients in the reference group had a higher proportion of strokes (18.7% vs. 13.1%; P=.004) and retinopathy (23.8% vs. 17.0%; P=.001).
Study patient characteristics.
| Study groups | Total | Metformin+other hypoglycemic agents | Metformin+DPP-4i | P |
| Number of patients, % | n=2067 | n=1548 | n=519 | |
| Demographic characteristics | ||||
| Mean age, years | 66.6 (10.9) | 67.3 (11.0) | 65.8 (10.4) | .007 |
| Gender (males) | 53.1% | 52.8% | 53.9% | NS |
| Pensioner scheme, SS | 77.8% | 80.7% | 69.4% | <.001 |
| General comorbidity | ||||
| Mean number of diagnoses | 5.5 (2.4) | 5.5 (2.5) | 5.3 (2.3) | NS |
| Charlson index mean | 1.0 (0.4) | 1.0 (0.4) | 1.0 (0.5) | NS |
| Mean RUB | 3.0 (0.6) | 3.0 (0.6) | 2.9 (0.5) | .047 |
| RUB-1 | 1.5% | 1.5% | 1.6% | |
| RUB-2 | 13.7% | 13.7% | 13.5% | |
| RUB-3 | 73.5% | 72.2% | 77.5% | |
| RUB-4 | 10.2% | 11.4% | 6.6% | |
| RUB-5 | 1.1% | 1.2% | 0.8% | .035 |
| Associated comorbidities | ||||
| High blood pressure | 68.3% | 69.1% | 65.9% | NS |
| Dyslipidemia | 63.8% | 63.7% | 64.0% | NS |
| Obesity | 25.2% | 24.8% | 26.2% | NS |
| Active smokers | 22.1% | 21.6% | 23.5% | NS |
| Alcoholism | 3.6% | 3.9% | 2.7% | NS |
| Ischemic heart disease | 15.2% | 16.0% | 12.9% | NS |
| Stroke | 17.3% | 18.7% | 13.1% | .004 |
| Organ failure: | 18.7% | 19.5% | 16.4% | NS |
| Bronchial asthma | 5.2% | 5.4% | 4.4% | NS |
| COPD | 6.9% | 6.8% | 6.9% | NS |
| Neuropathies | 1.0% | 1.0% | 0.8% | NS |
| Dementia (all types) | 3.4% | 3.6% | 2.7% | NS |
| Depressive syndrome | 20.6% | 19.6% | 23.7% | .044 |
| Malignant neoplasms | 10.3% | 10.7% | 8.9% | NS |
| Relationship with diabetes | ||||
| Time from diagnosis, years | 5.2 (3.5) | 5.4 (3.5) | 4.6 (3.2) | .001 |
| Diabetic retinopathy | 22.1% | 23.8% | 17.0% | .001 |
| Diabetic neuropathy | 3.4% | 3.6% | 3.1% | NS |
| Diabetic nephropathy | 2.2% | 2.3% | 1.7% | NS |
| Microalbuminuria | 15.7% | 16.9% | 12.1% | .010 |
Abbreviations: RUBs, resource utilization bands; COPD, chronic obstructive pulmonary disease; DPP-4i, dipeptidyl peptidase-4 inhibitors; NS, not significant; other hypoglycemic agents: including sulfonylureas, glitazones and insulin; P: statistical significance; SS: social security.
Values expressed in percentages or mean (standard deviation).
The patients treated with DPP-4i, in comparison with those that received other hypoglycemic agents, showed better treatment compliance (70.3% vs. 59.6%; P<.001) and treatment persistence (63.4% vs. 51.0%; P<.001). We achieved an acceptable correlation between the degree of compliance and the treatment time (r=0.481; P<.001). The metabolic control (HbA1c<7%) in the DPP-4i group at the completion of the 2-year follow-up was higher than in those treated with other hypoglycemic agents (64.3% vs. 59.6%, respectively; P<.001). Therapy with DPP-4i was associated with higher therapeutic compliance (OR=1.3; 95% CI: 1.1–1.5; P=.001), treatment duration (OR=1.2; 95% CI: 1.1–1.3; P=.032) and better metabolic control (OR=1.4; 95% CI: 1.2–1.6; P=.015).
The patients treated with DPP-4i, in comparison with those who received other hypoglycemic agents, attended fewer primary care medical visits (21.6% vs. 29.4%; P<.001), shorter hospital stays (0.1 vs. 0.2 days; P=.039) and fewer specialist care visits (1.8 vs. 2.6; P<.001).
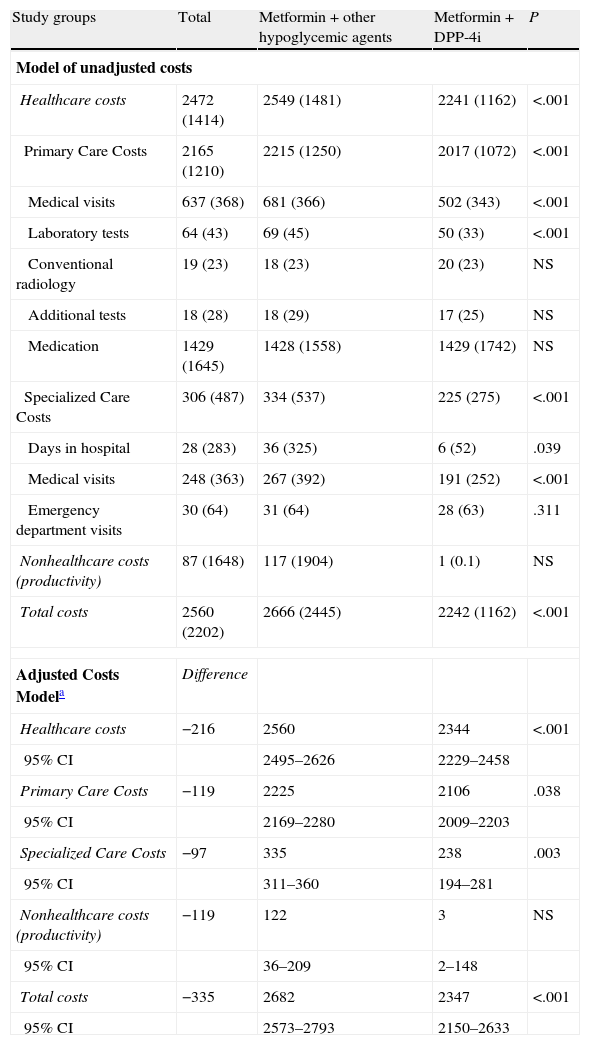
The total cost of care for patients with type 2 DM rose to 5.3 million euros, of which 96.6% corresponded to direct healthcare costs and 3.4% to indirect nonhealthcare costs (Table 3). The average/unit total costs (healthcare and nonhealthcare) of the subjects treated with DPP-4i was less than that calculated for patients who were treated with other hypoglycemic agents (2242 € vs. 2666 €; P<.001). In the adjusted model (ANCOVA), these costs were 2347 € (95% CI: 2.150–2.633 €) vs. 2682 € (95% CI: 2.573–2.793 €; P<.001). All components of the healthcare costs showed these differences (primary care and specialized). The nonhealthcare costs were not conclusive. The healthcare cost showed a moderate correlation with age (r=0.335) and general comorbidity (RUBs; r=0.348; P<.001).
Model of costs (net and adjusted) according to the study groups (average/unit in euros) during the 2-year follow-up.
| Study groups | Total | Metformin+other hypoglycemic agents | Metformin+DPP-4i | P |
| Model of unadjusted costs | ||||
| Healthcare costs | 2472 (1414) | 2549 (1481) | 2241 (1162) | <.001 |
| Primary Care Costs | 2165 (1210) | 2215 (1250) | 2017 (1072) | <.001 |
| Medical visits | 637 (368) | 681 (366) | 502 (343) | <.001 |
| Laboratory tests | 64 (43) | 69 (45) | 50 (33) | <.001 |
| Conventional radiology | 19 (23) | 18 (23) | 20 (23) | NS |
| Additional tests | 18 (28) | 18 (29) | 17 (25) | NS |
| Medication | 1429 (1645) | 1428 (1558) | 1429 (1742) | NS |
| Specialized Care Costs | 306 (487) | 334 (537) | 225 (275) | <.001 |
| Days in hospital | 28 (283) | 36 (325) | 6 (52) | .039 |
| Medical visits | 248 (363) | 267 (392) | 191 (252) | <.001 |
| Emergency department visits | 30 (64) | 31 (64) | 28 (63) | .311 |
| Nonhealthcare costs (productivity) | 87 (1648) | 117 (1904) | 1 (0.1) | NS |
| Total costs | 2560 (2202) | 2666 (2445) | 2242 (1162) | <.001 |
| Adjusted Costs Modela | Difference | |||
| Healthcare costs | −216 | 2560 | 2344 | <.001 |
| 95% CI | 2495–2626 | 2229–2458 | ||
| Primary Care Costs | −119 | 2225 | 2106 | .038 |
| 95% CI | 2169–2280 | 2009–2203 | ||
| Specialized Care Costs | −97 | 335 | 238 | .003 |
| 95% CI | 311–360 | 194–281 | ||
| Nonhealthcare costs (productivity) | −119 | 122 | 3 | NS |
| 95% CI | 36–209 | 2–148 | ||
| Total costs | −335 | 2682 | 2347 | <.001 |
| 95% CI | 2573–2793 | 2150–2633 | ||
Abbreviations: CI, confidence interval; DPP-4i, dipeptidyl peptidase-4 inhibitors; NS: not significant; other hypoglycemic agents: including sulfonylureas, glitazones and insulin; P: statistical significance.
Values expressed in mean (standard deviation).
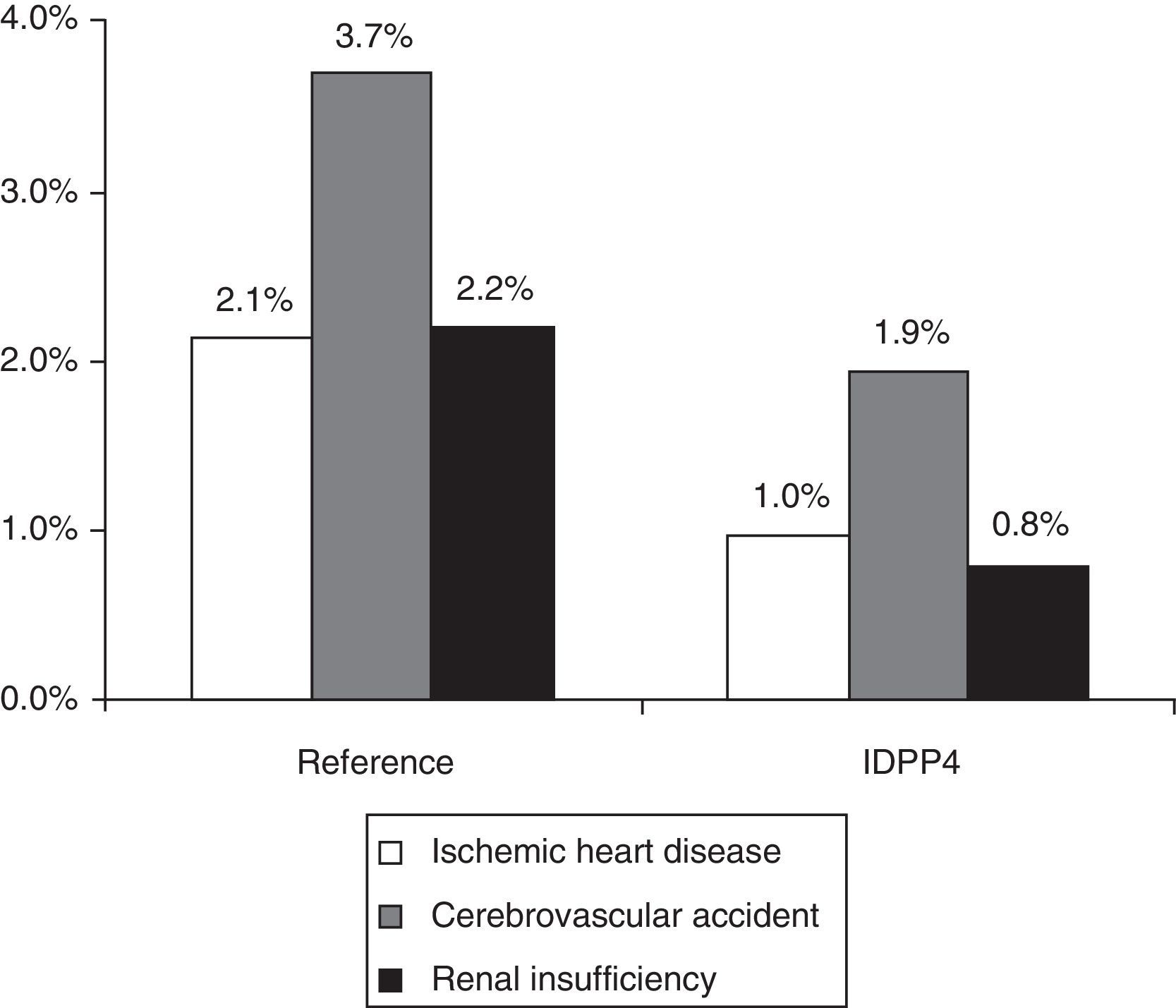
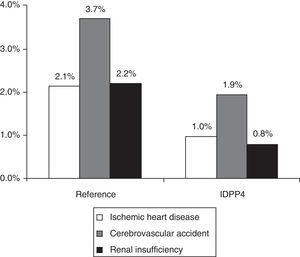
A total of 136 patients had some CVD (rate: 6.6%; 95% CI: 5.5%-7.7%). The rate of CVD in the group treated with DPP-4i, in comparison with those that were treated with other hypoglycemic agents, was lower (3.7% vs. 7.6%; P=.002). The group treated with DPP-4i had a lower proportion of new cases of ischemic heart disease (1.0% vs. 2.1%; P=.036), strokes (1.9% vs. 3.7%; P=.042) and renal failure (0.8% vs. 2.2%; P=.045) (Fig. 1). In the logistic regression model, the presence of CVD was associated with treatment non-compliance (OR=1.1; 95% CI: 1.0–1.8), poor control of the disease (OR=1.2; 95% CI: 1.1–1.7), overall comorbidity (OR=2.1; 95% CI: 1.6–2.9), male gender (OR=1.5; 95% CI: 1.1–2.2) and age (OR=1.1; 95% CI: 1.0–1.2) (P<.05). The percentage of patients with hypoglycemia was 36.7%. The patients of the DPP-4i group had a lower proportion of hypoglycemia than those who were treated with other hypoglycemic agents (13.9% vs. 44.3%; P<.001). Some 0.5% of the patients required hospitalization; 1.1% were treated in hospital emergency departments, and 35.7% were treated in Primary Care.
Distribution of cardiovascular events according to the study groups during the 2-year follow-up. Reference group: patients treated with metformin and another hypoglycemic drug (sulfonylurea, glitazone or insulin). DPP-4i group: patients treated with metformin and inhibitors of the dipeptidyl peptidase-4. Relationship of events: ischemic heart disease (n=38), stroke (n=67) and renal failure (n=38). The total number of events by group was n=107 and n=19 (P<.001), respectively. A patient could have more than one event. Renal failure: corresponds to a glomerular filtration rate <60mL/min. Statistically significant differences in the comparisons by pairs (P<.05).
The possible heterogeneity of the group treated with metformin with other hypoglycemic agents motivated a subanalysis of all drug groups (DPP-4i vs. sulfonylureas, glitazones and insulin). At the 2-year follow-up, the patients treated with DPP-4i had greater treatment compliance (70.3% vs. 59.9%, 60.3% and 58.4%, respectively); better control of the disease (64.3% vs. 62.6%, 62.8% and 50.5%, respectively) and a lower proportion of hypoglycemia (13.9% vs. 40.4%, 37.6% and 58.9%, respectively) (P<.001). The average/unit total costs were 2.242 € vs. 2.475 €, 2.724 € and 3.164 €, respectively (P<.001). The rates of CVD were 3.7% vs. 6.4%, 7.6 and 10.2%, respectively.
DiscussionOur study shows that patients treated with (double therapy) metformin and DPP-4i, when compared with those who were treated with metformin and other hypoglycemic agents including insulin, have a lower probability of experiencing hypoglycemia and, as a whole, are associated with lower healthcare costs. To a certain degree, it is possible that patients on insulin can interfere with the comparability of the group treated with metformin and other hypoglycemic agents (a study limitation). This is because they can be patients with more micro-macrovascular complications and/or with poorer metabolic control, probably due to presenting a type 2 DM that has progressed more or to being patients with greater genetic susceptibility. Nevertheless, their contribution to the full group of patients treated with metformin and other hypoglycemic agents is limited (18% of the total) and is in consistent with routine clinical practice, with these data consistent with those from other studies.19,20
DPP-4i was the second therapeutic option in this study population (25.1% of the total). Our results show that, at the 2-year follow-up, patients treated with DPP-4i show greater treatment compliance/persistence and better metabolic control. The studies on compliance and persistence with oral antidiabetic agents and insulin are scarce and difficult to compare with each other due to the different methodologies used. These studies show a compliance of 40%-80%. On this issue, Márquez Contreras et al.21 highlighted in a recent study that a quarter of diabetic patients do not comply with insulin therapy. Cramer et al.,22 in a review conducted on 139 studies, reported that at 12 months, the treatment persistence rate was 63% and the compliance with oral antidiabetic agents was 58%, which was similar in all analyzed therapeutic classes. Jermendy et al.23 in a series of patients in combination therapy with metformin and sulfonylureas, observed a 56% persistence rate at 1 year. Although these results are consistent with those found in our study, these variables were slightly higher in the patients treated with metformin and DPP-4i. This situation might be due to a random event (individual variability) or to the presence of a number of unidentified confounding factors. Nevertheless, a plausible explanation might be found in the presence of a better tolerance and safety profile and especially by a significantly lower rate of hypoglycemia.6,8 It seems clear that the role of DPP-4i in the therapeutic arsenal for type 2 DM is evolving swiftly, even though we lack long-term data to evaluate its effect on metabolic control. The available evidence about the direct association between compliance and metabolic control seems beyond all doubt.6,21,23
The second most relevant finding of this study is that the patients treated with metformin and DPP-4i had lower healthcare costs, with a reduction in the use of specialized care resources and fewer hospital admissions. The limited existing studies show that as compliance and metabolic control of these patients increase, the lower the risk of hospitalization. As an example, the review by Breitscheidel et al.24 concluded that improvements in compliance can lead to reduced total healthcare costs in type 2 DM. In 7 studies, compliance was inversely associated with total healthcare costs; the studies showed a lower cost due to a lower proportion of hospital days. Nevertheless, the variability of these studies is high. In general, our results are consistent with these studies,25 highlighting in particular the direct relationship between hypoglycemic episodes and a lower consumption of healthcare resources. Although the cost of DPP-4i agents is greater than that of alternative drugs, this cost seems offset by the lower costs of follow-up.
Our data show lower rates of CVD and renal failure in patients treated with DPP-4i. In this respect, evidence6–8,19,20 on both type 1 and type 2 DM has shown that good metabolic control results in significant improvements in the rate and outcomes of microangiopathic complications, a benefit that persists for years although it worsens metabolic control. The evolution of macrovascular complications, however, does not seem to depend as much on reaching an acceptable level of HbA1c, but rather on the type of treatment employed and the onset or not of hypoglycemic episodes. Given the close relationship between some microangiopathies (mainly nephropathy) and CVD, it is logical to consider that good metabolic control has a positively influence, but with a lower intensity than the control of other risk factors, such as dyslipidemia and high blood pressure.6,8,26 Currently, DPP-4i agents can have a cardiovascular benefit, but we have to wait for the results of various ongoing clinical trials.
The main limitation of this study is the possible selection bias by the attending physician when administering one or another hypoglycemic drug; the interpretation of the results should therefore be performed prudently. Similarly, it is possible that the patients who progress from metformin to insulin present higher complications. This situation, not measured exactly in our study, could be due to faster evolutionary forms of the disease and/or to the fact that patients treated with insulin present a greater degree of complexity. Another limitation of the study is due to the measurement of hypoglycemia, given that we only identified episodes in which the patient required medical care and in which this care was documented; episodes of hypoglycemia could therefore have been underdiagnosed. Additionally, the slight differences observed of greater age and progression time of the diabetes in the group treated with metformin and other hypoglycemic agents could partly explain the increased comorbidity and consumption of healthcare resources. Future research will be necessary in order to provide studies on cost-effectiveness and studies on diagnostic delay and treatment, and to collect data from other healthcare organizations. Despite the limitations of this study, the patients on treatment with DPP-4i (double therapy) showed an increased likelihood of greater compliance, metabolic control and lower rates of hypoglycemia, which resulted in lower healthcare costs.
Contribution of the authorsConcept and design of the manuscript: A. Sicras, C. Roldán and B. Font. Data collection: A. Sicras and R. Navarro. Interpretation of the data, drafting, review and approval of the submitted manuscript by all authors: A. Sicras, C. Roldán, B. Font, R. Navarro and J. Ibáñez.
FundingThe study was funded by Novartis Farmacéutica SA, who did not interfere in the final results.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sicras Mainar A, et al. Consecuencias clínicas y económicas de la combinación de metformina con inhibidores de la dipeptidilpeptidasa en pacientes con diabetes tipo 2. Rev Clin Esp. 2013;213:377–384.