Spain has been one of the countries most affected by the COVID-19 pandemic.
ObjectiveTo create a registry of patients with COVID-19 hospitalized in Spain in order to improve our knowledge of the clinical, diagnostic, therapeutic, and prognostic aspects of this disease.
DesignA multicenter retrospective cohort study that includes consecutive patients hospitalized with confirmed COVID-19 throughout Spain. Epidemiological and clinical data, additional tests at admission and at seven days, treatments administered, and progress at 30 days of hospitalization were collected from electronic medical records.
ResultsUp to June 30 2020, 15,111 patients from 150 hospitals were included. Their median age was 69.4 years (range: 18–102 years) and 57.2% were male. Prevalences of hypertension, dyslipidemia, and diabetes mellitus were 50.9%, 39.7%, and 19.4%, respectively. The most frequent symptoms were fever (84.2%) and cough (73.5%). High values of ferritin (73.5%), lactate dehydrogenase (73.9%), and D-dimer (63.8%) as well as lymphopenia (52.8%) were frequent. The most used antiviral drugs were hydroxychloroquine (85.6%) and lopinavir/ritonavir (61.4%). 33.1% developed respiratory distress. Overall mortality rate was 21.0%, with a marked increase with age (50-59 years: 4.7%, 60−69 years: 10.5%, 70−79 years: 26.9%, ≥80 years: 46.0%).
ConclusionThe SEMI-COVID-19 Network provides data on the clinical characteristics of patients with COVID-19 hospitalized in Spain. Patients with COVID-19 hospitalized in Spain are mostly severe cases, as one in three patients developed respiratory distress and one in five patients died. These findings confirm a close relationship between advanced age and mortality.
España ha sido uno de los países más afectados por la pandemia de COVID-19.
ObjetivoCrear un registro de pacientes hospitalizados en España por COVID-19 para mejorar nuestro conocimiento sobre los aspectos clínicos, diagnósticos, terapéuticos y pronósticos de esta enfermedad.
MétodosEstudio de cohorte retrospectiva, multicéntrico, que incluye pacientes consecutivos hospitalizados con COVID-19 confirmada en toda España. Se obtuvieron los datos epidemiológicos y clínicos, las pruebas complementarias al ingreso y a los siete días de la admisión, los tratamientos administrados y la evolución a los 30 días de hospitalización de las historias clínicas electrónicas.
ResultadosHasta el 30 de junio de 2020 se incluyeron 15.111 pacientes de 150 hospitales. Su mediana de edad fue 69,4 años (rango: 18-102 años) y el 57,2% eran hombres. Las prevalencias de hipertensión, dislipemia y diabetes mellitus fueron 50,9%, 39,7% y 19,4%, respectivamente. Los síntomas más frecuentes fueron fiebre (84,2%) y tos (73,5%). Fueron frecuentes los valores elevados de ferritina (73,5%), lactato deshidrogenasa (73,9%) y dímero D (63,8%), así como la linfopenia (52,8%). Los fármacos antivirales más utilizados fueron la hidroxicloroquina (85,6%) y el lopinavir/ritonavir (61,4%). El 33,1% desarrolló distrés respiratorio. La tasa de mortalidad global fue del 21,0%, con un marcado incremento con la edad (50-59 años: 4,7%, 60-69 años: 10,5%, 70-79 años: 26,9%, ≥80 años: 46%).
ConclusionesEl Registro SEMI-COVID-19 proporciona información sobre las características clínicas de los pacientes con COVID-19 hospitalizados en España. Los pacientes con COVID-19 hospitalizados en España son en su mayoría casos graves, ya que uno de cada tres pacientes desarrolló distrés respiratorio y uno de cada cinco pacientes falleció. Nuestros datos confirman una estrecha relación entre la edad avanzada y la mortalidad.
Spain is one of the countries with the highest number of patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the world. Since the first COVID-19 case was confirmed in the country on January 31, 2020, 253,908 cases have been diagnosed and 28,403 patients have died as of July 13, 2020.1
Current knowledge about COVID-19 is incomplete and fragmented. Cohort studies from various countries2–7 suggest that the risk factors and prognosis of this disease may not be able to be extrapolated to other geographical areas, as they could be influenced by specific public health conditions or race-related issues. To date, there are no solid therapeutic recommendations, as the results from ongoing clinical trials on the efficacy of antiviral and immunosuppressant drugs are pending.8–10
The SEMI-COVID-19 Network arises as an initiative of the Spanish Society of Internal Medicine (SEMI) to improve the quality of treatment for SARS-CoV-2. The main objective of the registry is to generate, in a short period of time, a large, multicenter cohort with detailed information on the epidemiology, clinical progress, and treatment received by patients. This will allow for the development of prognostic models and the assessment of the efficacy of different treatment regimens used in real-world clinical practice.
MethodsStudy designObservational studyThe SEMI-COVID Registry is an ongoing retrospective cohort comprising most consecutive patients with confirmed COVID-19 hospitalized and discharged in Spain from March 1, 2020 up to the end of the pandemic. Inclusion began on March 24 and is ongoing. Follow-up at one month was done via telephone.
Study population and participantsAll consecutive patients with confirmed SARS-COV-2 infection who had been discharged or died after hospital admission were eligible for inclusion. COVID-19 was confirmed either by a positive result on real-time polymerase chain reaction (RT-PCR) testing of a nasopharyngeal or sputum sample or by a positive result on serological testing and compatible clinical presentation.
Inclusion criteria for the registry were: a) patient age≥ 18 years, b) confirmed diagnosis of COVID-19, c) first hospital admission in a Spanish hospital participating in the study, d) hospital discharge or in-hospital death.
Exclusion criteria were subsequent admissions of the same patient and denial or withdrawal of informed consent.
Patients were treated at their attending physician’s discretion, according to local protocols and clinical judgement. Patients included in open-label clinical trials could be included in the registry, provided that all information about treatment was available. Given its observational nature, inclusion in the registry entailed no further inconvenience to the patients included.
Registry informationAn online electronic data capture system (DCS) has been developed, which includes a database manager along with procedures for the verification of data and contrasting of information against the original medical record in order to ensure the best possible quality of data collection.
Patient identifiable data are dissociated and pseudonymized. Direct identifiers are not collected in the DCS, but rather an alphanumeric sequence of characters that includes a code for identification of the researcher and a correlative number is used. Each researcher must maintain a protected registry (patient log) that is for his/her sole use. The purpose of this protected registry is to be able to confirm data with the medical records so that additional information may be gathered, if necessary, as well as to perform quality controls. This system allows for patient privacy to be respected, ethical considerations to be met, and data protection regulations to be complied with.
The database platform is hosted on a secure server. All information contained in the database, the configuration of the information within the database, as well as the database itself are fully encrypted. Every client-server data transfer is encrypted through a valid TLS certificate. Daily backups are performed in order to ensure data integrity.
Data collectionData are collected retrospectively and include approximately 300 variables grouped under various headings: (1) inclusion criteria, (2) epidemiological data, (3) RT-PCR and serology data, (4) personal medical and medication history, (5) symptoms and physical examination findings at admission, (6) laboratory (blood gases, metabolic panel, complete blood count, coagulation) and diagnostic imaging tests, (7) additional data at seven days after admission or at admission to the intensive care unit (ICU), (8) pharmacological treatment during the hospitalization (antiviral drugs, immunomodulators, antibiotics) and ventilatory support, (9) complications during the hospitalization, and (10) progress after discharge and/or 30 days from diagnosis. A list of variables can be found in Appendix A.
Study managementThe Spanish Society of Internal Medicine (SEMI, for its initials in Spanish) is the sponsor of this study. The researchers that coordinate the study from each hospital are SEMI members and were asked to participate in the study on a voluntary basis without receiving remuneration.
Database monitoring is performed by the study’s scientific steering committee and an independent external agency. Logistics coordination and data analysis are also carried out by external independent agencies.
Data analysisParticipating patients’ demographic, clinical, epidemiological, laboratory, and diagnostic imaging data were analyzed as well as their clinical progress. Quantitative variables are expressed as median [interquartile range]. Categorical variables are expressed as absolute frequencies and percentages. Mortality is expressed as case fatality rate (CFR).
Ethical aspectsPersonal data are processed in strict compliance with Spanish Law 14/2007, of July 3, on Biomedical Research; Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation); and Spanish Organic Law 3/2018, of December 5, on the Protection of Personal Data and the Guarantee of Digital Rights.
The SEMI-COVID-19 Registry has been approved by the Provincial Research Ethics Committee of Malaga (Spain).
In accordance with applicable regulations, the Spanish Agency of Medicines and Medical Products (AEMPS, for its initials in Spanish) has ruled that due to its nature, the study only required the approval of the Ethics Committee and not the Autonomous Community, as in other studies.
Informed consent was obtained from all the patients. When it was not possible to obtain informed consent in writing due to biosafety concerns or if the patient had already been discharged, informed consent was requested verbally and noted on the medical record.
The STROBE statement guidelines were followed in the conduct and reporting of the study.
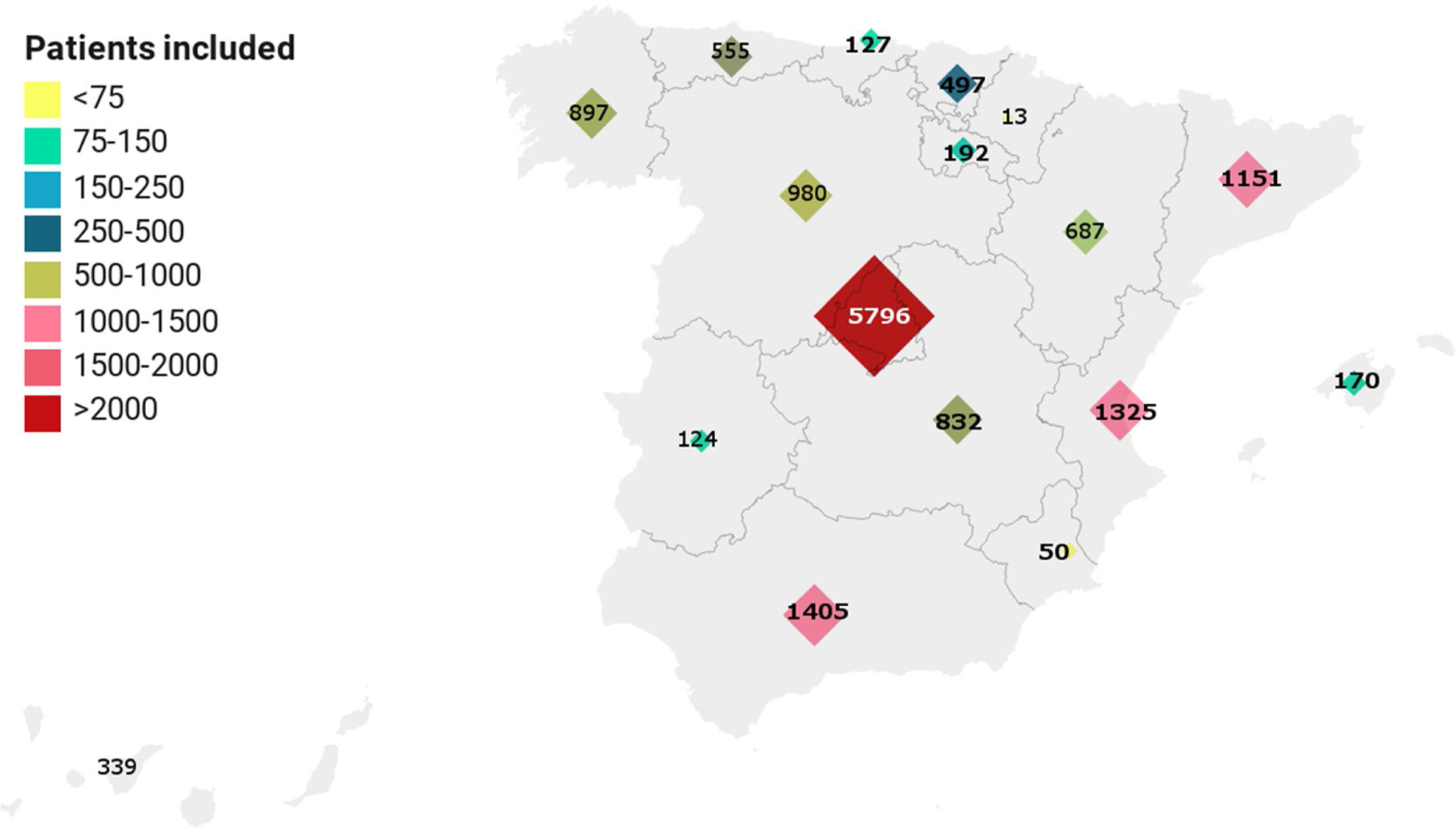
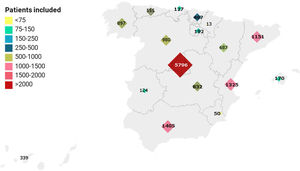
ResultsAs of June 30, 2020, 15111 patients hospitalized in 150 hospitals throughout Spain were included in the registry (Fig. 1). The epidemiological characteristics of population studied are described in Table 1. The median age was 69.4 years (range: 18–102 years) and 57.2% were male. Male gender was predominant in all age ranges except for patients ≥90 years, in which females accounted for 56.7% of the total.
Demographic and comorbidity data.
| Variable | Absolute frequency (%).*Median [Interquartilerange] | N |
|---|---|---|
| Age (years) | 69.4 [56.4;79.9] * | 15111 |
| 18−29 | 250 (1.7%) | |
| 30−64 | 6027 (39.9%) | |
| 65−79 | 5096 (33.7%) | |
| ≥80 | 3738 (24.7%) | |
| Gender | 15111 | |
| Male | 8643 (57.2%) | |
| Female | 6478 (42.8%) | |
| Race/Ethnicity | 14889 | |
| Caucasian | 13437 (90.2%) | |
| Other | 1452 (9.8%) | |
| Healthcare worker | 608 (4%) | 15093 |
| Age-adjusted Charlson Comorbidity Index | 14733 | |
| No comorbidities | 1753 (11.9%) | |
| Mild | 3927 (26.7%) | |
| Moderate | 4115 (27.9%) | |
| Severe | 4938 (33.5%) | |
| Degree of dependency | 14938 | |
| Independent or mild | 12460 (83.4%) | |
| Moderate | 1410 (9.4%) | |
| Severe | 1068 (7.1%) | |
| Tobacco use | 14419 | |
| Has never smoked | 9995 (69.3%) | |
| Former smoker | 3659 (25.4%) | |
| Smoker | 765 (5.3%) | |
| Alcohol use disorder | 690 (4.7%) | 14631 |
| Obesity (BMI ≥ 30 kg/m2) | 2910 (21.2%) | 13758 |
| Hypertension | 7689 (50.9%) | 15111 |
| Dyslipidemia | 5990 (39.7%) | 15104 |
| Diabetes mellitus | 2924 (19.4%) | 15095 |
| Cancer (solid tumor, leukemia, lymphoma) | 1610 (10.7%) | 15078 |
| Cardiovascular disease (atrial fibrillation, angina pectoris, heart failure) | 3001 (19.9%) | 15076 |
| Angina pectoris | 534 (3.5%) | 15107 |
| Atrial fibrillation | 1687 (11.2%) | 15095 |
| Heart failure | 1086 (7.2%) | 15107 |
| Myocardial infarction | 894 (5.9%) | 15111 |
| Obstructive lung disease (COPD, asthma) | 2071 (13.7%) | 15091 |
| COPD | 1038 (6.9%) | 15106 |
| Asthma | 1098 (7.3%) | 15101 |
| Obstructive sleep apnea/hypopnea syndrome | 903 (6.0%) | 15038 |
| Known HIV infection (with or without AIDS criteria) | 103 (0.7%) | 15075 |
| Moderate-severe chronic kidney disease | 917 (6.1%) | 15102 |
A high level of comorbidity was observed (61.4% with moderate or severe Charlson Comorbidity Index scores). Furthermore, 16.5% of patients had moderate or severe dependency for activities of daily living (Barthel index score <60). The most common comorbidities were hypertension (50.9%), dyslipidemia (39.7%), obesity (21.2%), and diabetes mellitus (19.4%).
Table 2 summarizes the clinical and radiological findings upon admission to the emergency department. The most common clinical manifestations were fever (84.2%), cough (73.5%), dyspnea (57.6%), and asthenia (43.6%). Anosmia, dysgeusia, and hyporexia were less common. Gastrointestinal manifestations were quite common, especially diarrhea. At triage, only 52.1% of patients were febrile and almost half showed some degree of respiratory failure (oxygen saturation <90% in 17.9%, respiratory rate >20 breaths per minute in 31.1%). Lung involvement was less common upon examination than in the radiographic findings: crepitant rales were present in 53.2% of patients whereas pneumonia or interstitial infiltrates were observed on chest X-rays in 86.8% of patients.
Clinical, laboratory, and diagnostic imaging findings upon admission.
| Variable | Absolute frequency (%). *Median [Interquartilerange] | N |
|---|---|---|
| Clinical presentation | ||
| Fever or low-grade fever | 15081 | |
| None | 2388 (15.8%) | |
| Low-grade fever (<38 °C) | 3131 (20.8%) | |
| Fever (> = 38 °C) | 9562 (63.4%) | |
| Cough | 15079 | |
| No | 3997 (26.5%) | |
| Yes, dry | 8751 (58%) | |
| Yes, with expectoration | 2331 (15.5%) | |
| Fatigue | 6507 (43.6%) | 14915 |
| Diarrhea | 3554 (23.7%) | 14991 |
| Anorexia | 2915 (19.6%) | 14845 |
| Shortness of breath | 8684 (57.6%) | 15067 |
| Anosmia | 1040 (7.1%) | 14710 |
| Physical Examination | ||
| Oxygen saturation (pulse oximetry, %) | 94 [91;97] | 14705 |
| <90 | 2628 (17.9%) | |
| ≥ 90 | 12077 (82.1%) | |
| Oxygen saturation/FiO2 ratio (%) | 442.9 [404.8;457.1] * | 14411 |
| Temperature, ºC | 37 [36.3;37.8] * | 14646 |
| <37 °C | 7026 (48%) | |
| 37-37.9 °C | 4520 (30.9%) | |
| ≥ 38 °C | 3100 (21.2%) | |
| Hypotension (systolic blood pressure <100 mmHg) | 907 (6.3%) | 14464 |
| Tachycardia (>100 beats per minute) | 3751 (24.8%) | 15140 |
| Tachypnea (>20 breaths per minute) | 4590 (31.1%) | 14769 |
| Confusion | 1803 (12%) | 14992 |
| Crackles | 7854 (53.2%) | 14754 |
| Chest x-ray | 14949 | |
| No pulmonary infiltrates | 1973 (13.2%) | |
| Unilateral pulmonary infiltrates | 3058 (20.5%) | |
| Bilateral pulmonary infiltrates | 9918 (66.3%) | |
| Complete blood count | ||
| White blood cell count, (x106/L) | 6300 [4780;8520] * | 15015 |
| Absolute count (x106/L) | ||
| Neutrophils | 4600 [3200;6700] * | 14944 |
| Lymphocytes | 940 [690;1300] * | 14990 |
| >1200 | 4818 (32.1%) | |
| 1000-1200 | 2249 (15%) | |
| 800-1000 | 2729 (18.2%) | |
| <800 | 5194 (34.6%) | |
| Eosinophils | 0 [0;20] * | 14786 |
| Monocytes | 400 [300;600] * | 14866 |
| Hemoglobin (g/dL) | 13.9 [12.6;15] * | 15016 |
| Platelets (x106/L) | 190000 [148000;247000] * | 15012 |
| Arterial Blood Gases | ||
| pH | 7.5 [7.4;7.5] * | 7764 |
| PCO2 (mmHg) | 34 [30.7;39] * | 7851 |
| PO2 (mmHg) | 66 [56;77.6] * | 7509 |
| pO2/FiO2 ratio (%) | 288.6 [233.3;342.9] * | 7203 |
| Basic metabolic panel | ||
| Glucose (mg/dL) | 112 [98;136] * | 14547 |
| Serum creatinine (mg/dL) | 0.9 [0.7;1.2] * | 14977 |
| Urea (mg/dL) | 37 [27;55] * | 12095 |
| Lactate dehydrogenase (U/L) | 321 [246;432] * | 13053 |
| <250 | 3410 (26.1%) | |
| 250-400 | 5634 (43.2%) | |
| >400 | 4009 (30.7%) | |
| Aspartate aminotransferase (U/L) | 35 [25;52] * | 11974 |
| Alanine aminotransferase (U/L) | 29 [19;46] * | 14145 |
| C-reactive protein (mg/L) | 60.2 [19;127.9] * | 14483 |
| Lactate (mmol/L) | 1.6 [1.1;2.4] * | 6824 |
| Procalcitonin (ng/mL) | 0.1 [0.1;0.2] * | 7159 |
| Interleukin-6 (IL-6) (pg/mL) | 29.8 [11.5;65.4] * | 1993 |
| D-dimer (ng/mL) | 11749 | |
| < 500 | 4251 (36.2%) | |
| 500-1000 | 3610 (30.7%) | |
| > 1000 | 3888 (33.1%) | |
| Serum ferritin (μg/L) | 5978 | |
| <300 | 1584 (26.5%) | |
| 300-650 | 1583 (26.5%) | |
| >650 | 2811 (47%) | |
| qSOFA index | 0 [0;1] * | 14129 |
| Low risk ≥1 | 12817 (90.7%) | |
| High risk ≥2 | 1312 (9.3%) | |
Laboratory findings at admission are also shown in Table 2. Decreased lymphocytes and eosinophil counts were of note: the median values were 940 and 0 × 106/L, respectively. High lactate dehydrogenase (LDH), D-dimer, and ferritin levels were observed in 73.9%, 63.8%, and 73.5%, respectively.
Treatment and complications during hospitalization are summarized in Table 3. A wide variety of drugs with purported antiviral effects have been used, the most frequent of which were hydroxychloroquine (85.6%) and lopinavir/ritonavir (61.4%). Remdesivir was only used in 68 patients (0.5%). Antibiotics were also widely indicated, mainly beta-lactam antibiotics (71.7%) and azithromycin (60.8%). Immunomodulatory drugs were also common, principally corticosteroids (35.2%), beta-interferon (11.3%), and tocilizumab (8.4%). Low-molecular-weight heparin was used in 83.4% of patients, generally at prophylactic doses.
Treatment and complications during hospitalization.
| Variable | Absolute frequency (%) | N |
|---|---|---|
| Antimicrobial therapy | ||
| Hydroxychloroquine | 12915 (85.6%) | 15084 |
| Lopinavir/Ritonavir (LPV/r) | 9254 (61.4%) | 15072 |
| Azithromycin | 9146 (60.8%) | 15036 |
| Beta-lactam antibiotics | 10795 (71.7%) | 15050 |
| Remdesivir | 68 (0.5%) | 14968 |
| Immunomodulatory therapy | ||
| Systemic corticosteroids | 5287 (35.2%) | 15034 |
| Interferon Beta-1B (IFNb) | 1689 (11.3%) | 15008 |
| Tocilizumab | 1276 (8.5%) | 15038 |
| Anakinra | 91 (0.6%) | 14939 |
| Immunoglobulin | 70 (0.5%) | 14821 |
| Ventilatory support | ||
| High flow nasal cannula | 1197 (8.0%) | 14989 |
| Invasive mechanical ventilation (IMV) | 998 (6.6%) | 15057 |
| Non-invasive mechanical ventilation (NIMV) | 733 (4.9%) | 15051 |
| Anticoagulant therapy | ||
| Low-molecular-weight heparin during hospitalization | 15016 | |
| No | 2645 (17.6%) | |
| Low (prophylactic) dose | 9713 (64.7%) | |
| High (anticoagulant) dose | 1648 (11%) | |
| Intermediate dose | 1010 (6.7%) | |
| Complications | ||
| Acute respiratory distress syndrome (ARDS) | 15057 | |
| No | 10077 (66.9%) | |
| Mild | 1203 (8.0%) | |
| Moderate | 1097 (7.3%) | |
| Severe | 2680 (17.8%) | |
| Bacterial pneumonia | 1680 (11.1%) | 15075 |
| Sepsis | 937 (6.2%) | 15080 |
| Intensive Care Unit admission | 1255 (8.3%) | 15129 |
| Outcome | ||
| Discharge | 11928 (78.8%) | 15140 |
| Death | 3181 (21.0%) | 15140 |
| Readmission | 573 (3.9%) | 14709 |
| Not discharged at the end of follow-up (after readmission) | 31 (0.2%) | 15140 |
Many patients required support: high flow nasal cannula was used in 8.0% of patients, noninvasive positive-pressure ventilation in 4.9%, and invasive mechanical ventilation in 6.6%. The main complication was acute respiratory distress syndrome (ARDS), which 33.1% of patients developed, followed by bacterial pneumonia and sepsis. Although 2680 patients developed severe ARDS, only 1255 (8.3%) were transferred to an intensive care unit.
The median follow-up period was 40 days (range: 0–102 days). At the end of follow-up, 78.8% had been discharged, 21.0% had died, and 0.2% continued hospitalized (after readmission). The average length of hospital stay for discharged patients was 10.4 days (range: 1–62 days). The rate of readmission within 30 days was 3.9% (573 patients).
DiscussionIn this study, we analyze a large series of patients hospitalized with COVID-19 in Spain who have been included in the SEMI-COVID-19 Registry. This first cohort includes consecutive patients admitted to hospitals throughout Spain who were discharged or died. Similar to almost all Western series, our patients were predominantly male, elderly, and with multiple comorbidities.
Recently, the first conclusions about the impact of COVID-19 in Madrid, the epicenter of the pandemic in Spain, were drawn from a large cohort of 2226 patients from La Paz University Hospital of Madrid.11 The strengths and weaknesses of this study both arise from its single-center design: the data are more consistent and able to be analyzed, but are also less able to be extrapolated to the general population and prone to local biases, such as different population demographics or features specific to that particular hospital.
Our series has a higher proportion of males, as has been described in most multicenter cohorts and contrary to the work by Borobia et al.11 The higher proportion of females at La Paz University Hospital may be a result of the specific demographic features of its reference population and thus does not reflect the differences according to sex previously described in other viral infections in general and specifically in COVID-19.
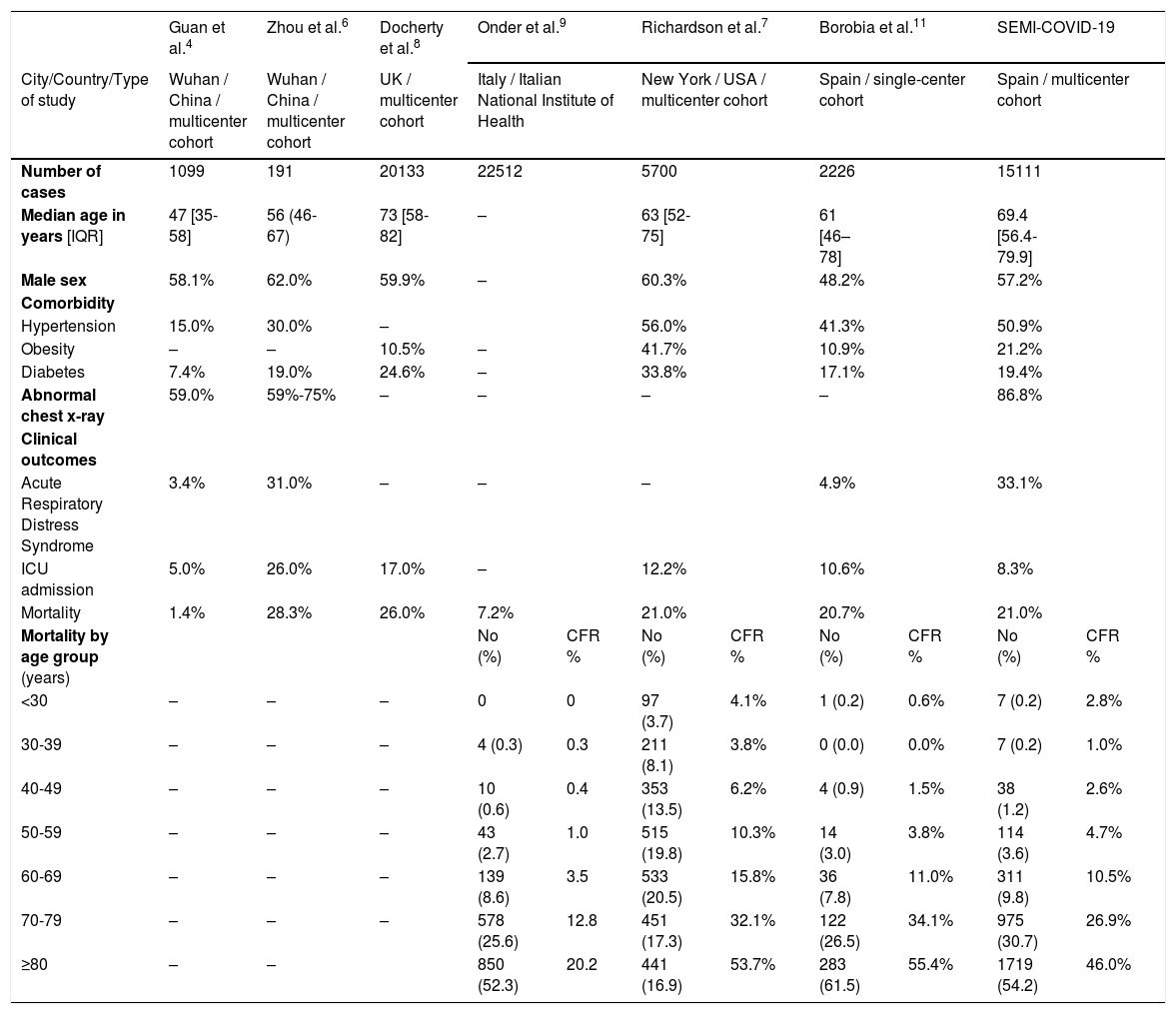
In addition, our cohort includes older patients with a greater number of comorbidities. In our series, the median age was 69 years (61 in Madrid cohort11), which is clearly higher than Guan et al.’s Chinese series,4 moderately higher than Richardson et al.’s New York series,7 and lower than Docherty et al.’s UK series.8 The most frequent comorbidities (hypertension, diabetes, obesity, dementia, and others) are similar to those that have been previously described, but all were more prevalent among our patients. They are summarized in Table 4.
Comparison of baseline characteristics and outcome of patients with COVID-19 included in series from different countries.
| Guan et al.4 | Zhou et al.6 | Docherty et al.8 | Onder et al.9 | Richardson et al.7 | Borobia et al.11 | SEMI-COVID-19 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| City/Country/Type of study | Wuhan / China / multicenter cohort | Wuhan / China / multicenter cohort | UK / multicenter cohort | Italy / Italian National Institute of Health | New York / USA / multicenter cohort | Spain / single-center cohort | Spain / multicenter cohort | ||||
| Number of cases | 1099 | 191 | 20133 | 22512 | 5700 | 2226 | 15111 | ||||
| Median age in years [IQR] | 47 [35-58] | 56 (46-67) | 73 [58-82] | – | 63 [52-75] | 61 [46–78] | 69.4 [56.4-79.9] | ||||
| Male sex | 58.1% | 62.0% | 59.9% | – | 60.3% | 48.2% | 57.2% | ||||
| Comorbidity | |||||||||||
| Hypertension | 15.0% | 30.0% | – | 56.0% | 41.3% | 50.9% | |||||
| Obesity | – | – | 10.5% | – | 41.7% | 10.9% | 21.2% | ||||
| Diabetes | 7.4% | 19.0% | 24.6% | – | 33.8% | 17.1% | 19.4% | ||||
| Abnormal chest x-ray | 59.0% | 59%-75% | – | – | – | – | 86.8% | ||||
| Clinical outcomes | |||||||||||
| Acute Respiratory Distress Syndrome | 3.4% | 31.0% | – | – | – | 4.9% | 33.1% | ||||
| ICU admission | 5.0% | 26.0% | 17.0% | – | 12.2% | 10.6% | 8.3% | ||||
| Mortality | 1.4% | 28.3% | 26.0% | 7.2% | 21.0% | 20.7% | 21.0% | ||||
| Mortality by age group (years) | No (%) | CFR % | No (%) | CFR % | No (%) | CFR % | No (%) | CFR % | |||
| <30 | – | – | – | 0 | 0 | 97 (3.7) | 4.1% | 1 (0.2) | 0.6% | 7 (0.2) | 2.8% |
| 30-39 | – | – | – | 4 (0.3) | 0.3 | 211 (8.1) | 3.8% | 0 (0.0) | 0.0% | 7 (0.2) | 1.0% |
| 40-49 | – | – | – | 10 (0.6) | 0.4 | 353 (13.5) | 6.2% | 4 (0.9) | 1.5% | 38 (1.2) | 2.6% |
| 50-59 | – | – | – | 43 (2.7) | 1.0 | 515 (19.8) | 10.3% | 14 (3.0) | 3.8% | 114 (3.6) | 4.7% |
| 60-69 | – | – | – | 139 (8.6) | 3.5 | 533 (20.5) | 15.8% | 36 (7.8) | 11.0% | 311 (9.8) | 10.5% |
| 70-79 | – | – | – | 578 (25.6) | 12.8 | 451 (17.3) | 32.1% | 122 (26.5) | 34.1% | 975 (30.7) | 26.9% |
| ≥80 | – | – | 850 (52.3) | 20.2 | 441 (16.9) | 53.7% | 283 (61.5) | 55.4% | 1719 (54.2) | 46.0% | |
IQR: interquartile range; ICU: intensive care unit; CFR%: case fatality rate percentage: SEMI: Spanish Society of Internal Medicine.
In our cohort, the main symptoms reported upon admission (fever, cough, dyspnea, and asthenia) were similar to those reported in other studies,4–8 although myalgia and anosmia were less common. This could potentially be explained by a difference in admission criteria: patients without lung involvement were managed as outpatients from emergency departments and, therefore, only the most severe cases were admitted.
In our series, mortality, as defined by CFR, was similar to what was observed in the Madrid cohort,11 some Chinese series,2–6 and the USA cohort,7 but was much higher than the Italian cohort9 and lower than what has been described in the UK.8
The difference between our series and the Italian series warrants some explanation, as we share many demographic features with Italy and the timing and magnitude of the COVID-19 pandemic have been similar. The difference in mortality may reflect different study inclusion criteria or different hospital admission criteria. Less strict admission or inclusion criteria yield a greater number of patients included in the registry, thus lowering the CFR. Indeed, population-based studies, which include more patients with milder disease, have lower CFRs than hospital-centered series.9 Conversely, stricter admission or inclusion criteria lead to greater severity among the patients analyzed and an increase in the CFR.
Another explanation could be that these observational works could not control for factors related to race, including the percentage and origin of immigrant populations or healthcare-system disparities. In fact, racial and demographic factors may in part explain the differences in severity and mortality between Chinese and Western series.2–8
Demographic factors, such as age or comorbidities, may partially explain the differences in mortality and can be controlled for by means of multivariate analysis. Pressure on the healthcare system can result in different mortality rates, as was shown in China by Liang et al.,12 who compared the CFR both within and outside of Hubei province (CFR of 7.3% vs. 0.3%, respectively).
In Italy,9 the pandemic placed the greatest pressure on the region of Lombardy whereas in Spain, it has been more widely distributed. Nevertheless, the majority of patients in our series are from hospitals in Madrid, which has been one of the most affected regions and where the situation is comparable to that of northern Italy. Whether there is a geographical influence will be further explored in additional studies.
As has been shown in all series, a high percentage of patients had abnormal laboratory values that were consistent with an abnormal inflammatory profile.2–8 In our series, lymphopenia and elevated levels of D-dimer, LDH, and ferritin were the most frequent findings. Also, a large part of our patients received treatment that has purported antiviral activity against SARS-CoV-2. Our multicenter registry has been designed to allow for multivariate analysis of the prognostic value of these abnormal laboratory findings as well as treatment received during hospitalization.
Notably, in our series, there was a much higher proportion of patients with ARDS (moderate or severe: 25.1% or 3777 patients) than patients who were admitted to an ICU (8.3%, 1255 patients). This suggests that only approximately one out of every three patients with ARDS was admitted to an ICU.
We have discussed this finding in detail and have evaluated some possible confounding factors and biases. On the one hand, patients admitted directly to an ICU or who died in an ICU may have not been included in our cohort and thus altered our ICU admission rate. Patients who have still not been discharged have not been included in our cohort. Therefore, patients who are currently hospitalized in the ICU thus also falsely lower our ICU admission rate. Patients with ARDS may have died before being transferred to an ICU or have presented with criteria that is not compatible with treatment in an ICU, but even still, this does not explain how 2522 out of 3777 patients with moderate or severe ARDS were discharged without having been admitted to an ICU.
Another plausible explanation could be the overloading of the healthcare system, at least in the most affected regions of the country. It is known that the number of ICU beds has increased substantially during the COVID-19 pandemic in Spain. It is likely that in addition to increasing the number of ICU beds, some semi-intensive care areas were established within hospitals. In our personal experience, many hospitals have designed “semi-intensive” or “intermediate care” wards in order to provide ventilatory support to patients when ICU expansion was no longer feasible. This finding warrants further examination.
The collaborative effort of the SEMI-COVID-19 Network Group has provided us with a large amount of data from a sizeable number of patients. Among the strengths of our registry are its multicenter design; its wide geographical dispersion, which limits local biases (selection, admission, treatment availability, ICU availability, etc.) and increases its external validity; and its large size, which provides statistical power for confirming hypotheses.
This study also has limitations. First, data are collected by a large number of researchers from different centers, which could lead to heterogeneity in data collection and validation. Second, the registry includes consecutive patients from participating centers, which limits patient selection bias but introduces another selection bias according to participating centers. Third, our registry, though extensive (more than 300 variables), collects only basic data for enhancing our knowledge of COVID-19, but lacks the level of detail required for deeper analysis of very specific aspects. Lastly, the main limitation of this study is its observational design, which does not allow for establishing causal relationships.
This is the largest reported series of hospitalized patients in Spain with confirmed COVID-19 disease and one of the largest registries in the world to date. Though our findings are currently preliminary and must be explored in greater detail, the SEMI-COVID-19 Network working group and the SEMI-COVID-19 Registry will surely become a key tool for helping clinicians and researchers improve knowledge of this novel disease which has threatened not only the lives of many patients and the proper functioning of our healthcare systems, but also the foundations of our economy and way of life.
Funding informationThe Spanish Society of Internal Medicine (SEMI, for its initials in Spanish) is the sponsor of this study. This work has not received any specific funding from public, commercial, or non-profit entities.
Conflicts of interest disclosureThe authors declare that there are no conflicts of interest.
We wholeheartedly thank all the investigators who participate in the SEMI-COVID-19 Network Group. We also thank the SEMI-COVID-19 Registry Coordination Center, S&H Medical Science Service, for their data quality control and logistic and administrative support.
Coordinator of the SEMI-COVID-19 Registry: José Manuel Casas Rojo.
SEMI-COVID-19 Scientific Committee Members: José Manuel Casas Rojo, José Manuel Ramos Rincón, Carlos Lumbreras Bermejo, Jesús Millán Núñez-Cortés, Juan Miguel Antón Santos, Ricardo Gómez Huelgas.
SEMI-COVID-19 Registry Coordinating Center: S & H Medical Science Service.
Members of the SEMI-COVID-19 Group:
H. U. 12 de Octubre. Madrid. Paloma Agudo de Blas, Coral Arévalo Cañas, Blanca Ayuso, José Bascuñana Morejón, Samara Campos Escudero, María Carnevali Frías, Santiago Cossío Tejido, Borja de Miguel Campo, Carmen Díaz Pedroche, Raquel Díaz Simón, Ana García Reyne, Lucia Jorge Huerta, Antonio Lalueza Blanco, Jaime Laureiro Gonzalo, Carlos Lumbreras Bermejo, Guillermo Maestro de la Calle, Bárbara Otero Perpiña, Diana Paredes Ruiz, Marcos Sánchez Fernández, Javier Tejada Montes. H. U. Gregorio Marañón. Madrid. Laura Abarca Casas, Álvaro Alejandre de Oña, Rubén Alonso Beato, Leyre Alonso Gonzalo, Jaime Alonso Muñoz, Christian Mario Amodeo Oblitas, Cristina Ausín García, Marta Bacete Cebrián, Jesús Baltasar Corral, María Barrientos Guerrero, Alejandro Bendala Estrada, María Calderón Moreno, Paula Carrascosa Fernández, Raquel Carrillo, Sabela Castañeda Pérez, Eva Cervilla Muñoz, Agustín Diego Chacón Moreno, María Carmen Cuenca Carvajal, Sergio de Santos, Andrés Enríquez Gómez, Eduardo Fernández Carracedo, María Mercedes Ferreiro-Mazón Jenaro, Francisco Galeano Valle, Alejandra García, Irene García Fernández-Bravo, María Eugenia García Leoni, María Gómez Antúnez, Candela González San Narciso, Anthony Alexander Gurjian, Lorena Jiménez Ibáñez, Cristina Lavilla Olleros, Cristina Llamazares Mendo, Sara Luis García, Víctor Mato Jimeno, Clara Millán Nohales, Jesús Millán Núñez-Cortés, Sergio Moragón Ledesma, Antonio Muiño Miguez, Cecilia Muñoz Delgado, Lucía Ordieres Ortega, Susana Pardo Sánchez, Alejandro Parra Virto, María Teresa Pérez Sanz, Blanca Pinilla Llorente, Sandra Piqueras Ruiz, Guillermo Soria Fernández-Llamazares, María Toledano Macías, Neera Toledo Samaniego, Ana Torres do Rego, María Victoria Villalba García, Gracia Villarreal, María Zurita Etayo. H. Universitari de Bellvitge. L’Hospitalet de Llobregat. Xavier Corbella, Abelardo Montero, José María Mora-Luján. C. H. U. de Albacete. Albacete. José Luis Beato Pérez, María Lourdes Sáez Méndez. H. U. La Paz-Cantoblanco-Carlos III. Madrid. Jorge Álvarez Troncoso, Francisco Arnalich Fernández, Francisco Blanco Quintana, Carmen Busca Arenzana, Sergio Carrasco Molina, Aranzazu Castellano Candalija, Germán Daroca Bengoa, Alejandro de Gea Grela, Alicia de Lorenzo Hernández, Alejandro Díez Vidal, Carmen Fernández Capitán, María Francisca García Iglesias, Borja González Muñoz, Carmen Rosario Herrero Gil, Juan María Herrero Martínez, Víctor Hontañón, María Jesús Jaras Hernández, Carlos Lahoz, Cristina Marcelo Calvo, Juan Carlos Martín Gutiérrez, Mónica Martínez Prieto, Elena Martínez Robles, Araceli Menéndez Saldaña, Alberto Moreno Fernández, José María Mostaza Prieto, Ana Noblejas Mozo, Carlos Manuel Oñoro López, Esmeralda Palmier Peláez, Marina Palomar Pampyn, María Angustias Quesada Simón, Juan Carlos Ramos Ramos, Luis Ramos Ruperto, Aquilino Sánchez Purificación, Teresa Sancho Bueso, Raquel Sorriguieta Torre, Clara Itziar Soto Abanedes, Yeray Untoria Tabares, Marta Varas Mayoral, Julia Vásquez Manau. Complejo Asistencial de Segovia. Segovia. Eva María Ferreira Pasos, Daniel Monge Monge, Alba Varela García. H. U. Puerta de Hierro. Majadahonda. Madrid. María Álvarez Bello, Ane Andrés Eisenhofer, Ana Arias Milla, Isolina Baños Pérez, Javier Bilbao Garay, Silvia Blanco Alonso, Jorge Calderón Parra, Alejandro Callejas Díaz, José María Camino Salvador, Mª Cruz Carreño Hernández, Valentín Cuervas-Mons Martínez, Sara de la Fuente Moral, Miguel del Pino Jiménez, Alberto Díaz de Santiago, Itziar Diego Yagüe, Ignacio Donate Velasco, Ana María Duca, Pedro Durán del Campo, Gabriela Escudero López, Esther Expósito Palomo, Ana Fernández Cruz, Esther Fiz Benito, Andrea Fraile López, Amy Galán Gómez, Sonia García Prieto, Claudia García Rodríguez-Maimón, Miguel Ángel García Viejo, Javier Gómez Irusta, Edith Vanessa Gutiérrez Abreu, Isabel Gutiérrez Martín, Ángela Gutiérrez Rojas, Andrea Gutiérrez Villanueva, Jesús Herráiz Jiménez, Pedro Laguna del Estal, Mª Carmen Máinez Sáiz, Cristina Martín Martín, María Martínez Urbistondo, Fernando Martínez Vera, Susana Mellor Pita, Patricia Mills Sánchez, Esther Montero Hernández, Alberto Mora Vargas, Cristina Moreno López, Alfonso Ángel-Moreno Maroto, Víctor Moreno-Torres Concha, Ignacio Morrás De La Torre, Elena Múñez Rubio, Ana Muñoz Gómez, Rosa Muñoz de Benito, Alejandro Muñoz Serrano, José María Palau Fayós, Ilduara Pintos Pascual, Antonio Ramos Martínez, Isabel Redondo Cánovas del Castillo, Alberto Roldán Montaud, Lucía Romero Imaz, Yolanda Romero Pizarro, Mónica Sánchez Santiuste, David Sánchez Ortiz, Enrique Sánchez Chica, Patricia Serrano de la Fuente, Pablo Tutor de Ureta, Ángela Valencia Alijo, Mercedes Valentín-Pastrana Aguilar, Juan Antonio Vargas Núñez, José Manuel Vázquez Comendador, Gema Vázquez Contreras, Carmen Vizoso Gálvez. H. Miguel Servet. Zaragoza. Gonzalo Acebes Repiso, Uxua Asín Samper, María Aranzazu Caudevilla Martínez, José Miguel García Bruñén, Rosa García Fenoll, Jesús Javier González Igual, Laura Letona Giménez, Mónica Llorente Barrio, Luis Sáez Comet. H. U. La Princesa. Madrid. María Aguilera García, Ester Alonso Monge, Jesús Álvarez Rodríguez, Claudia Álvarez Varela, Miquel Berniz Gòdia, Marta Briega Molina, Marta Bustamante Vega, José Curbelo, Alicia de las Heras Moreno, Ignacio Descalzo Godoy, Alexia Constanza Espiño Álvarez, Ignacio Fernández Martín-Caro, Alejandra Franquet López-Mosteiro, Gonzalo Gálvez Márquez, María J. García Blanco, Yaiza García del Álamo Hernández, Clara García-Rayo Encina, Noemí Gilabert González, Carolina Guillamo Rodríguez, Nicolás Labrador San Martín, Manuel Molina Báez, Carmen Muñoz Delgado, Pedro Parra Caballero, Javier Pérez Serrano, Laura Rabes Rodríguez, Pablo Rodríguez Cortés, Carlos Rodríguez Franco, Emilia Roy-Vallejo, Mónica Rueda Vega, Aresio Sancha Lloret, Beatriz Sánchez Moreno, Marta Sanz Alba, Jorge Serrano Ballester, Alba Somovilla, Carmen Suarez Fernández, Macarena Vargas Tirado, Almudena Villa Martí. H. U. de A Coruña. A Coruña. Alicia Alonso Álvarez, Olaya Alonso Juarros, Ariadna Arévalo López, Carmen Casariego Castiñeira, Ana Cerezales Calviño, Marta Contreras Sánchez, Ramón Fernández Varela, Santiago J. Freire Castro, Ana Padín Trigo, Rafael Prieto Jarel, Fátima Raad Varea, Laura Ramos Alonso, Francisco Javier Sanmartín Pensado, David Vieito Porto. H. Clínico San Carlos. Madrid. Inés Armenteros Yeguas, Javier Azaña Gómez, Julia Barrado Cuchillo, Irene Burruezo López, Noemí Cabello Clotet, Alberto E. Calvo Elías, Elpidio Calvo Manuel, Carmen María Cano de Luque, Cynthia Chocron Benbunan, Laura Dans Vilan, Ester Emilia Dubon Peralta, Vicente Estrada Pérez, Santiago Fernández-Castelao, Marcos Oliver Fragiel Saavedra, José Luis García Klepzig, Maria del Rosario Iguarán Bermúdez, Esther Jaén Ferrer, Rubén Ángel Martín Sánchez, Manuel Méndez Bailón, Maria José Nuñez Orantos, Carolina Olmos Mata, Eva Orviz García, David Oteo Mata, Cristina Outon González, Juncal Pérez-Somarriba, Pablo Pérez Mateos, Maria Esther Ramos Muñoz, Xabier Rivas Regaira, Iñigo Sagastagoitia Fornie, Alejandro Salinas Botrán, Miguel Suárez Robles, Maddalena Elena Urbano, Miguel Villar Martínez. H. Infanta Sofía. S. S. de los Reyes. Madrid. Rafael del Castillo Cantero, Rebeca Fuerte Martínez, Arturo Muñoz Blanco, José Francisco Pascual Pareja, Isabel Perales Fraile, Isabel Rábago Lorite, Llanos Soler Rangel, Inés Suárez García, José Luis Valle López. H. U. Dr. Peset. Valencia. Juan Alberto Aguilera Ayllón, Arturo Artero Mora, María del Mar Carmona Martín, María José Fabiá Valls, María de Mar Fernández Garcés, Ana Belén Gómez Belda, Ian López Cruz, Manuel Madrazo López, Elisabeth Mateo Sanchís, Jaume Micó Gandía, Laura Piles Roger, Adela María Pina Belmonte, Alba Viana García. H. Clínico de Santiago. Santiago de Compostela. María del Carmen Beceiro Abad, María Aurora Freire Romero, Sonia Molinos Castro, Emilio Manuel Páez Guillan, María Pazo Núñez, Paula María Pesqueira Fontán. H. U. Ramón y Cajal. Madrid. Luis Fernando Abrego Vaca, Ana Andréu Arnanz, Octavio Arce García, Marta Bajo González, Pablo Borque Sanz, Alberto Cozar Llisto, Sonia de Pedro Baena, Beatriz Del Hoyo Cuenda, María Alejandra Gamboa Osorio, Isabel García Sánchez, Andrés González García, Oscar Alberto López Cisneros, Miguel Martínez Lacalzada, Borja Merino Ortiz, Jimena Rey-García, Elisa Riera González, Cristina Sánchez Díaz, Grisell Starita Fajardo, Cecilia Suárez Carantoña, Adrián Viteri Noel, Svetlana Zhilina Zhilina. C. Asistencial de Zamora. Zamora. Carlos Aldasoro Frías, Luis Arribas Pérez, María Esther Fraile Villarejo, Beatriz García López, Víctor Madrid Romero, Emilia Martínez Velado, Victoria Palomar Calvo, Sara Pintos Otero, Carlota Tuñón de Almeida. H. Royo Villanova. Zaragoza. Nicolás Alcalá Rivera, Anxela Crestelo Vieitez, Esther del Corral, Jesús Díez Manglano, Isabel Fiteni Mera, María del Mar García Andreu, Martín Gericó Aseguinolaza, Claudia Josa Laorden, Raúl Martínez Murgui, Marta Teresa Matía Sanz. H. U. Infanta Cristina. Parla. Madrid. Juan Miguel Antón Santos, Ana Belén Barbero Barrera, Coralia Bueno Muiño, Ruth Calderón Hernáiz, Irene Casado López, José Manuel Casas Rojo, Andrés Cortés Troncoso, Mayte de Guzmán García-Monge, Francesco Deodati, Gonzalo García Casasola Sánchez, Elena García Guijarro, Davide Luordo, María Mateos González, José A Melero Bermejo, Lorea Roteta García, Elena Sierra Gonzalo, Javier Villanueva Martínez. H. de Cabueñes. Gijón. Ana María Álvarez Suárez, Carlos Delgado Vergés, Rosa Fernández-Madera Martínez, Eva Fonseca Aizpuru, Alejandro Gómez Carrasco, Cristina Helguera Amezua, Juan Francisco López Caleya, María del Mar Martínez López, Aleida Martínez Zapico, Carmen Olabuenaga Iscar, María Luisa Taboada Martínez, Lara María Tamargo Chamorro. H. de Urduliz Alfredo Espinosa. Urdúliz. María Aparicio López, Asier Aranguren Arostegui, Paula Arriola Martínez, Gorka Arroita González, Mª Soledad Azcona Losada, Miriam García Gómez, Eduardo García López, Amalur Iza Jiménez, Alazne Lartategi Iraurgi, Esther Martínez Becerro, Itziar Oriñuela González, Isabel María Portales Fernández, Pablo Ramírez Sánchez, Beatriz Ruiz Estévez, Cristian Vidal Núñez. H. Regional Universitario de Málaga. Málaga. M. Mar Ayala Gutiérrez, Rosa Bernal López, José Bueno Fonseca, Verónica Andrea Buonaiuto, Luis Francisco Caballero Martínez, Lidia Cobos Palacios, Clara Costo Muriel, Francis de Windt, Ana Teresa Fernández-Truchaud Christophel, Paula García Ocaña, Ricardo Gómez Huelgas, Javier Gorospe García, María Dolores López Carmona, Pablo López Quirantes, Almudena López Sampalo, Elizabeth Lorenzo Hernández, Juan José Mancebo Sevilla, Jesica Martin Carmona, Luis Miguel Pérez-Belmonte, Araceli Pineda Cantero, Michele Ricci, Jaime Sanz Cánovas. H. Santa Marina. Bilbao. María Areses Manrique, Ainara Coduras Erdozain, Ane Elbire Labirua-Iturburu Ruiz. H. Ntra. Sra. del Prado. Talavera de la Reina. Toledo. Sonia Casallo Blanco, Jeffrey Oskar Magallanes Gamboa. H. HLA Universitario Moncloa. Madrid. Guillermo Estrada, Teresa García Delange, Isabel Jiménez Martínez, Carmen Martínez Cilleros, Nuria Parra Arribas. H. del Henares. Coslada. Madrid. Jesús Ballano Rodríguez-Solís, Luis Cabeza Osorio, María del Pilar Fidalgo Montero, M. Isabel Fuentes Soriano, Erika Esperanza Lozano Rincón, Ana Martín Hermida, Jesús Martínez Carrilero, José Ángel Pestaña Santiago, Manuel Sánchez Robledo, Patricia Sanz Rojas, Nahum Jacobo Torres Yebes, Vanessa Vento. H. U. Torrevieja. Torrevieja. Alicante. Julio César Blázquez Encinar. H. U. La Fe. Valencia. Dafne Cabañero, María Calabuig Ballester, Pascual Císcar Fernández, Ricardo Gil Sánchez, Marta Jiménez Escrig, Cristina Marín Amela, Laura Parra Gómez, Carlos Puig Navarro, José Antonio Todolí Parra. H. San Pedro. Logroño. Diana Alegre González, Irene Ariño Pérez de Zabalza, Sergio Arnedo Hernández, Jorge Collado Sáenz, Beatriz Dendariena, Marta Gómez del Mazo, Iratxe Martínez de Narvajas Urra, Sara Martínez Hernández, Estela Menéndez Fernández, José Luis Peña Somovilla, Elisa Rabadán Pejenaute. H. U. Virgen del Rocío. Sevilla. Verónica Alfaro Lara, Bosco Barón Franco, Máximo Bernabeu-Wittel, Concepción Conde Guzmán, Juan Delgado de la Cuesta, Pablo Díaz Jiménez, Fátima Espinosa Torre, Rosa María Gámez Mancera, Luis Giménez Miranda, Aurora González Estrada, Sonia Gutiérrez Rivero, Carlos Hernández Quiles, Carlos Jiménez de Juan, Julia Lanseros Tenllado, María del Carmen López Ríos, María Nieto, Santiago Rodríguez Suárez, Jara Eloisa Ternero Vega. H. U. Ntra. Sra. Candelaria. Santa Cruz de Tenerife. Lucy Abella, Andrea Afonso Díaz, Selena Gala Aguilera García, Marta Bethencourt Feria, Eduardo Mauricio Calderón Ledezma, Sara Castaño Pérez, Guillermo Castro Gainett, José Manuel del Arco Delgado, Joaquín Delgado Casamayor, Diego García Silvera, Alba Gómez Hidalgo, Marcelino Hayek Peraza, Carolina Hernández Carballo, Rubén Hernández Luis, Francisco Javier Herrera Herrera, María del Mar López Gámez, Julia Marfil Daza, María José Monedero Prieto, María Blanca Monereo Muñoz, María de la Luz Padilla Salazar, Daniel Rodríguez Díaz, Alicia Tejera, Laura Torres Hernández. H. U. San Juan de Alicante. San Juan de Alicante. David Balaz, David Bonet Tur, Carles García Cervera, David Francisco García Núñez, Vicente Giner Galvañ, Angie Gómez Uranga, Javier Guzmán Martínez, Isidro Hernández Isasi, Lourdes Lajara Villar, Juan Manuel Núñez Cruz, Sergio Palacios Fernández, Juan Jirge Peris García, Andrea Riaño Pérez, José Miguel Seguí Ripoll, Philip Wikman-Jorgensen. H. U. San Agustín. Avilés. Andrea Álvarez García, Víctor Arenas García, Alba Barragán Mateos, Demelsa Blanco Suárez, María Caño Rubia, Jaime Casal Álvarez, David Castrodá Copa, José Ferreiro Celeiro, Natalia García Arenas, Raquel García Noriega, Joaquín Llorente García, Irene Maderuelo Riesco, Paula Martínez García, María José Menéndez Calderón, Diego Eduardo Olivo Aguilar, Marta Nataya Solís Marquínez, Luis Trapiella Martínez, Andrés Astur Treceño García, Juan Valdés Bécares. H. de Sagunto. Sagunto. Valencia. Zineb Karroud Zamrani, José Maréa Pascual Izuel, Enrique Rodilla Sala. H. de Mataró. Mataró. Raquel Aranega González, Ramon Boixeda, Carlos Lopera Mármol, Marta Parra Navarro, Ainhoa Rex Guzmán, Aleix Serrallonga Fustier. H. U. Son Llàtzer. Palma de Mallorca. Andrés de la Peña Fernández, Almudena Hernández Milián. H. Juan Ramón Jiménez. Huelva. Francisco Javier Bejarano Luque, Francisco Javier Carrasco-Sánchez, Mercedes de Sousa Baena, Jaime Díaz Leal, Aurora Espinar Rubio, María Franco Huertas, Juan Antonio García Bravo, Andrés González Macías, Encarnación Gutiérrez Jiménez, Alicia Hidalgo Jiménez, Constantino Lozano Quintero, Carmen Mancilla Reguera, Francisco Javier Martínez Marcos, Francisco Muñoz Beamud, María Pérez Aguilera, Alicia Pérez Jiménez, Virginia Rodríguez Castaño, Álvaro Sánchez de Alcázar del Río, Leire Toscano Ruiz. H. U. Reina Sofía. Córdoba. Antonio Pablo Arenas de Larriva, Pilar Calero Espinal, Javier Delgado Lista, María Jesús Gómez Vázquez, José Jiménez Torres, Laura Martín Piedra, Javier Pascual Vinagre, María Elena Revelles Vílchez, Juan Luis Romero Cabrera, José David Torres Peña. H. Moisès Broggi. Sant Joan Despí. José Loureiro Amigo, Melani Pestaña Fernández, Nicolas Rhyman, Nuria Vázquez Piqueras. H. U. C. de Asturias. Oviedo. Víctor Asensi Álvarez, Itxasne Cabezón Estévanez, María Folgueras Gómez, María Martínez Sela, Lucía Meijide Rodríguez, Claudia Moran Castaño, Noelia Morán Suárez, Sara Rodríguez Suárez, Silvia Suárez Díaz, Lucia Suárez Pérez, Carlos Vázquez, Carmen Yllera Gutiérrez. H. U. Virgen de las Nieves. Granada. Pablo Conde Baena, Joaquín Escobar Sevilla, Laura Gallo Padilla, Patricia Gómez Ronquillo, Pablo González Bustos, María Navío Botías, Jessica Ramírez Taboada, Mar Rivero Rodríguez. H. San Juan de la Cruz. Úbeda. Marcos Guzmán García, Francisco Javier Vicente Hernández. H. Costa del Sol. Marbella. Málaga. Victoria Agustín Bandera, María Dolores Martín Escalante. H. Infanta Margarita. Cabra. María Esther Guisado Espartero, Lorena Montero Rivas, María de la Sierra Navas Alcántara, Raimundo Tirado-Miranda. Complejo Asistencial Universitario de León. León. Rosario María García Die, Manuel Martin Regidor, Ángel Luis Martínez González, Alberto Muela Molinero, Raquel Rodríguez Díez, Beatriz Vicente Montes. Hospital Clínic Barcelona. Júlia Calvo Jiménez, Aina Capdevila Reniu, Irene Carbonell De Boulle, Emmanuel Coloma Bazán, Joaquim Fernández Solà, Cristina Gabara Xancó, Joan Ribot Grabalosa, Olga Rodríguez Núñez. Hospital Marina Baixa. Villajoyosa. Alicante. Javier Ena, Santiago Pérez Martín. C. H. U. de Ferrol. Ferrol. Hortensia Álvarez Díaz, Tamara Dalama López, Estefanía Martul Pego, Carmen Mella Pérez, Ana Pazos Ferro, Sabela Sánchez Trigo, Dolores Suarez Sambade, María Trigas Ferrin, María del Carmen Vázquez Friol, Laura Vilariño Maneiro. H. Insular de Gran Canaria. Las Palmas G. C. Carlos Jorge Ripper. H. del Tajo. Aranjuez. Madrid. Ruth González Ferrer, Raquel Monsalvo Arroyo. H. U. Marqués de Valdecilla. Santander. Marta Fernández-Ayala Novo, José Javier Napal Lecumberri, Nuria Puente Ruiz, José Riancho, Isabel Sampedro García. H. Torrecárdenas. Almería. Luis Felipe Díez García, Iris El Attar Acedo, Bárbara Hernández Sierra, Carmen Mar Sánchez Cano. H. U. Severo Ochoa. Leganés. Yolanda Casillas Viera, Lucía Cayuela Rodríguez, Carmen de Juan Álvarez, Gema Flox Benitez, Laura García Escudero, Juan Martin Torres, Patricia Moreira Escriche, Susana Plaza Canteli, M Carmen Romero Pérez. H. Platón. Barcelona. Ana Suarez Lombraña. H. Asepeyo Coslada. Coslada. Madrid. Alejo Erice Calvo-Sotelo. Hospital Valle del Nalón. Riaño (Langreo). Sara Fuente Cosío, César Manuel Gallo Álvaro, Julia Lobo García, Antía Pérez Piñeiro. H. U. del Vinalopó. Elche. Francisco Amorós Martínez, Erika Ascuña Vásquez, José Carlos Escribano Stablé, Adriana Hernández Belmonte, Ana Maestre Peiró, Raquel Martínez Goñi, M. Carmen Pacheco Castellanos, Bernardino Soldan Belda, David Vicente Navarro. H. Alto Guadalquivir. Andújar. Begoña Cortés Rodríguez. H. Francesc de Borja. Gandía. Valencia. Alba Camarena Molina, Simona Cioaia, Anna Ferrer Santolalia, José María Frutos Pérez, Eva Gil Tomás, Leyre Jorquer Vidal, Marina Llopis Sanchís, Mari Ángeles Martínez Pascual, Álvaro Navarro Batet, Mari Amparo Perea Ribis, Ricardo Peris Sánchez, José Manuel Querol Ribelles, Silvia Rodríguez Mercadal, Ana Ventura Esteve. H. G. U. de Castellón. Castellón de la Plana. Jorge Andrés Soler, Marián Bennasar Remolar, Alejandro Cardenal Álvarez, Daniela Díaz Carlotti, María José Esteve Gimeno, Sergio Fabra Juana, Paula García López, María Teresa Guinot Soler, Daniela Palomo de la Sota, Guillem Pascual Castellanos, Ignacio Pérez Catalán, Celia Roig Martí, Paula Rubert Monzó, Javier Ruiz Padilla, Nuria Tornador Gaya, Jorge Usó Blasco. H. Santa Bárbara. Soria. Marta León Téllez. C. A. U. de Salamanca. Salamanca. Gloria María Alonso Claudio, Víctor Barreales Rodríguez, Cristina Carbonell Muñoz, Adela Carpio Pérez, María Victoria Coral Orbes, Daniel Encinas Sánchez, Sandra Inés Revuelta, Miguel Marcos Martín, José Ignacio Martín González, José Ángel Martín Oterino, Leticia Moralejo Alonso, Sonia Peña Balbuena, María Luisa Pérez García, Ana Ramón Prados, Beatriz Rodríguez-Alonso, Ángela Romero Alegría, María Sánchez Ledesma, Rosa Juana Tejera Pérez. H. Virgen de la Salud. Toledo. Ana María Alguacil Muñoz, Marta Blanco Fernández, Verónica Cano, Ricardo Crespo Moreno, Fernando Cuadra García-Tenorio, Blanca Díaz-Tendero Nájera, Raquel Estévez González, María Paz García Butenegro, Alberto Gato Díez, Verónica Gómez Caverzaschi, Piedad María Gómez Pedraza, Julio González Moraleja, Raúl Hidalgo Carvajal, Patricia Jiménez Aranda, Raquel Labra González, Áxel Legua Caparachini, Pilar López Castañeyra, Agustín Lozano Ancín, José Domingo Martin García, Cristina Morata Romero, María Jesús Moya Saiz, Helena Moza Moríñigo, Gemma Muñiz Nicolás, Enriqueta Muñoz Platón, Elena Ortiz Ortíz, Raúl Perea Rafael, Pilar Redondo Galán, María Antonia Sepúlveda Berrocal, Pilar Toledano Sierra, Jesús Vázquez Clemente, Carmen Yera Bergua. H. U. de Canarias. Santa Cruz de Tenerife. Julio Cesar Alvisa Negrín, José Fernando Armas González, Lourdes González Navarrete, Iballa Jiménez, María Candelaria Martín González, Miguel Nicolás Navarrete Lorite, Paula Ortega Toledo, Onán Pérez Hernández, Alina Pérez Ramírez. H. de Poniente. Almería. Juan Antonio Montes Romero, Encarna Sánchez Martín, José Luis Serrano Carrillo de Albornoz, Manuel Jesús Soriano Pérez. H. Sierrallana. Torrelavega. Cristina Amado Fernández, Tomás de Vega Santos, Cristina Limia, Lucia Paz Fajardo, Andrea Tejero Fernández, Reina Valle Bernad. H. U. Lucus Augusti. Lugo. Raquel Gómez Méndez, Ana Rodríguez Álvarez. H. San Pedro de Alcántara. Cáceres. Ángela Agea García, Javier Galán González, Luis Gámez Salazar, Eva García Sardón, Antonio González Nieto, Itziar Montero Días, Selene Núñez Gaspar, Álvaro Santaella Gómez. H. U. del Sureste. Arganda del Rey. Madrid. Jon Cabrejas Ugartondo, Ana Belén Mancebo Plaza, Arturo Noguerado Asensio, Bethania Pérez Alves, Natalia Vicente López. H. de Pozoblanco. Pozoblanco. José Nicolás Alcalá Pedrajas, Antonia Márquez García, Inés Vargas. H. Virgen de los Lirios. Alcoy (Alicante). María José Esteban Giner. H. Doctor José Molina Orosa. Arrecife (Lanzarote). Virginia Herrero García, Berta Román Bernal. H. Nuestra Señora de Sonsoles. Ávila. Alaaeldeen Abdelhady Kishta. C. H. U. de Badajoz. Badajoz. Rafael Aragón Lara, Inmaculada Cimadevilla Fernández, Juan Carlos Cira García, Gema María García García, Julia González Granados, Beatriz Guerrero Sánchez, Francisco Javier Monreal Periáñez, María Josefa Pascual Pérez. Hospital de Palamós. Palamós. Anabel Martin-Urda Diez-Canseco. H. G. U. de Elda. Elda. Carmen Cortés Saavedra, Jennifer Fernández Gómez, Borja González López, María Soledad Hernández Garrido, Ana Isabel López Amorós, María de los Reyes Pascual Pérez, Andrea Torregrosa García. H. U. Puerta del Mar. Cádiz. José Antonio Girón González, Susana Fabiola Pascual Pérez, Cristina Rodríguez Fernández-Viagas, María José Soto Cardenas. H. de Montilla. Montilla. Ana Cristina Delgado Zamorano, Beatriz Gómez Marín, Adrián Montaño Martínez, José Luis Zambrana García. H. Infanta Elena. Huelva. María Gloria Rojano Rivero. H. U. Quironsalud Madrid.Pozuelo de Alarcón (Madrid). Pablo Guisado Vasco, Ana Roda Santacruz, Ana Valverde Muñoz. H. de la Axarquía. Vélez- Málaga. Antonio López Ruiz. H. Virgen del Mar. Madrid. Thamar Capel Astrua, Paola Tatiana García Giraldo, María Jesús González Juárez, Victoria Márquez Fernández, Ada Viviana Romero Echevarry. H. do Salnes. Vilagarcía de Arousa. Vanesa Alende Castro, Ana María Baz Lomba, Ruth Brea Aparicio, Marta Fernández Morales, Jesús Manuel Fernández Villar, María Teresa López Monteagudo, Cristina Pérez García, Lorena María Rodríguez Ferreira, María Begoña Valle Feijoo. H. Parc Taulí. Sabadell. Francisco Epelde, Isabel Torrente. H. Clínico Universitario de Valladolid. Valladolid. Pablo Tellería Gómez. H. Quironsalud A Coruña. A Coruña. Héctor Meijide Miguez. Fundación Hospital Calahorra. Calahorra (La Rioja). Jesús Castiella Herrero. H. U. Rafael Méndez. Lorca. Ana Isabel Peláez Ballesta. H. La Fuenfría. Cercedilla. Isabel Rodríguez Fraile. H. U. Infanta Leonor. Madrid. Beatriz Mestre Gómez. H. P. de Monforte de Lemos. Monforte de Lemos. Manuel Lorenzo López Reboiro. H. U. de la Plana. Vila-Real (Castellón). Lorena Pérez Pérez. Consorci Sanitari de Terrassa. Terrassa. Anna Fajardo Modol. H. Perpetuo Socorro. Badajoz. María José Luque Calderón. H. de Éibar. Éibar. Esperanza Montero Aparicio. H. Joan March. Bunyola (Mallorca). Cristina Gallego Lezaun. H. Comarcal de Blanes. Blanes. Pere Comas Casanova. H. Santa Ana. Motril. Granada. Jesús Palomares Rodríguez. H. de Zafra. Zafra. Juana Carretero Gómez. H. G. U. Los Arcos del Mar Menor. San Javier. Diana Piñar Cabezos. H. García Orcoyen. Estella. María del Carmen Martínez Velasco. H. U. de Gran Canaria Dr. Negrín. Las Palmas G. C. Alicia Conde. C. H. U. de Cáceres. Cáceres. Marta Correa Matos. H. de Barbastro. Barbastro. Juan Salas Jarque. Fundació Sant Hospital de la Seu d’Urgell. La Seu d’Urgell. Luis Enrique Cajamarca Calva. H. C. Medina del Campo. Medina del Campo. David Morchón Simón. H. U. Santa Lucía. Cartagena. Pedro José García López. H. Comarcal de Inca. Inca. María Soledad Sanz Parras. H. de Barbanza. Ribeira. Lara María Mateo Mosquera. H. G. U. Reina Sofía. Murcia. José Joaquín Hernández Roca. H. del Vendrell. El Vendrell. Ana Lacal Martínez. H. U. Río Hortega. Valladolid. Luis Corral Gudino. H. U. Virgen de la Victoria. Málaga. María José Benítez Toledo. H. U. Rey Juan Carlos. Móstoles. José Antonio Rueda Camino. H. San Juan de Dios del Aljarafe. Bormujos. Ana Laura Blanco Taboada. Centro Médico de Asturias. Oviedo. Fidel Asensio Fierro. Clínica San Miguel. Pamplona. Pamplona. Raquel Rodil.
IAS Sta. Caterina. Salt. Sara García Torras. H. Insular Ntra. Sra. de los Reyes. Valverde (El Hierro). Ana María Torres Vega. Complejo Hospitalario Universitario Ourense. Ourense. Amara González Noya. H. Germans Trias i Pujol. Badalona. Elia Fernández Pedregal. H. U. Santa Cristina. Madrid. Juan Gallego Galiana.
Please cite this article as: Casas-Rojo JM, Antón-Santos JM, Millán-Núñez-Cortés J, Lumbreras-Bermejo C, Ramos-Rincón JM, Roy-Vallejo E et al.. Características clínicas de los pacientes hospitalizados con COVID-19 en España: resultados del Registro SEMI-COVID-19. Rev Clin Esp. 2020;220:480–494.