To study the prevalence of neutralizing antibodies in healthcare workers and healthcare support personnel after the administration of the second dose of the BNT162b2 vaccine (Pfizer-BioNTech).
Materials and MethodsIn December 2021, we undertook a study in the Health Department in Orihuela, Alicante (Spain), which consists of 1500 workers. We collected demographic variables about the study participants, and we performed a “point-of-care” immunochromatography test to measure the presence of neutralizing antibodies (OJABIO® SARS-CoV-2 Neutralizing Antibody Detection Kit, manufactured by Wenzhou OJA Biotechnology Co., Ltd. Wenzhou, Zhejiang, China) before the administration of the third dose of the vaccine.
ResultsWe obtained complete information about 964 (64%) workers, which consisted of 290 men and 674 women. The average age was 45,8 years (min. 18, max. 68) and the average time since the last dose of the vaccine was 40,5 weeks (min. 1,71, max. 47,71). A total of 131 participants (13,5%) had suffered infection by SARS-CoV-2 confirmed using RT-PCR. The proportion of participants who showed presence of neutralizing antibodies was 38,5%. In the multivariable analysis, the time since the last dose of the vaccine (aOR week: 1,07; 95%CI: 1,04; 1,09) and previous infection by SARS-CoV-2 (aOR: 3,7; 95CI: 2,39; 5,63) showed a statistically significant association with the presence of neutralizing antibodies.
ConclusionsThe time since the administration of the last dose of the vaccine and the previous infection by SARS-CoV-2 determined the presence of neutralizing antibodies in 38,5% of the healthcare workers and support workers.
Estudiar la prevalencia de anticuerpos neutralizantes en el personal sanitario y personal sanitario de apoyo tras la administración de la segunda dosis de vacuna BNT162b2 (Pfizer–BioNTech).
MethodsEn diciembre 2021 llevamos a cabo un estudio en el Departamento de Salud de Orihuela, Alicante (España), formado por 1500 trabajadores. En los participantes del estudio, recogimos variables demográficas y realizamos un test “point-of-care” de inmunocromatografía para medir la presencia de anticuerpos neutralizantes (OJABIO® SARS-CoV-2 Neutralizing Antibody Detection Kit, fabricado por Wenzhou OJA Biotechnology Co., Ltd.- Wenzhou, Zhejiang, China) antes de la administración de la tercera dosis de vacuna.
ResultadosObtuvimos información completa de 964 (64%) trabajadores, siendo 290 varones y 674 mujeres. La edad media fue de 45,8 años (mín: 18, max: 68) y el tiempo desde la última dosis de vacuna fue 40,5 semanas (mín: 1,71; máx: 47,71). Un total de 131 (13,5%) habían padecido infección por SARS-CoV-2 confirmada mediante RT-PCR. La proporción de sujetos con presencia de anticuerpos neutralizantes fue 38,5%. En el análisis multivariable el tiempo desde la última dosis de vacuna (ORa semana: 1.07; 95%CI: 1,04; 1,09) y la infección previa por SARS-CoV-2 (ORa: 3.7; 95%CI: 2,39; 5,63) mostraron asociación estadísticamente significativa con la presencia de anticuerpos neutralizantes.
ConclusionesEl tiempo desde la administración de la última dosis de vacuna y la infección previa por SARS-CoV-2 determinaron la presencia de anticuerpos neutralizantes en 38,5% del personal sanitario y personal de apoyo.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine has been one of the main tools for preventing COVID-19 disease.1 In Spain, mass SARS-CoV-2 vaccination campaigns were conducted in December 2020. Healthcare professionals and healthcare support personnel were considered front-line groups during the COVID-19 pandemic and were included in the priority risk group in vaccination strategies. The main vaccine used in this group was the BNT162b2 (Pfizer-BioNTech) vaccine.2 The data reported on this vaccine by some clinical trials and real-world studies3,4 have shown high efficacy (94%–95%) against symptomatic cases of moderate-to-severe disease after administration of the second dose.3–6
Although the efficacy of the SARS-CoV-2 vaccine has been widely demonstrated by multiple studies, reaching protection rates of up to 95% after the second dose,3,4 it seems that the long-term durability of the immunological protection is limited. Some studies report a decrease in vaccine efficacy related to increased hospitalization and a greater mortality rate at 20 weeks after vaccination with two doses of the ChAdOx1-S or BNT162b2 vaccine.5–7 In addition, time has also been associated with a decrease in neutralizing antibody titers against SARS-CoV-2.8–10
The B.1.617.2 (delta) variant became the predominant variant as of August 2021, with PCR sequencing frequencies of between 50%–100%.11 During this period, an increase in the incidence of SARS-CoV-2 infection and a decrease in vaccination coverage were observed. Both aspects together call the efficacy of vaccines marketed against emerging variants into question.12,13 Nonetheless, mRNA vaccines have been shown to be safe and effective against severe infection, hospitalization, and death caused by different SARS-CoV-2 variants, including the delta variant.14 Even so, aspects related to the immunogenicity associated with the neutralizing activity of the antibodies generated must still be clarified15 and further studies are needed to evaluate this activity in the long term.
A wide variety of diagnostic tools have been used in order to understand the extent of SARS-CoV-2 propagation and the population immunity status as well as to promote the containment and mitigation of the COVID-19 pandemic.16–18 Regarding the techniques used for the serological analysis, tests based on lateral flow immunochromatography (LFIC) have been shown to be tools capable of detecting anti-SARS-CoV-2 antibodies, such as anti-S protein antibodies, in a rapid, practical, and economically accessible manner.17,18
IgG antibodies against SARS-CoV-2 S or “spike” protein (receptor binding domain (RBD) fragment) have demonstrated a good correlation with the titer of neutralizing antibodies against SARS-CoV-2.9,17 The S protein is the antigenic target of most vaccines because it acts as a pathway for the virus to enter the cell. The mutational evolution of this protein in the different emerging SARS-CoV-2 variants compromises the neutralizing capacity of the antibodies generated after vaccination, making it necessary to re-evaluate and redesign the marketed vaccines.18,19
Therefore, COVID-19 disease remains a challenge at present. Prevention and diagnostic strategies continue to be necessary in order to avoid an uptick in SARS-CoV-2 infection. In addition to being cheaper and faster (around 15 min) than other routine serological assays, LFIC tests have been tested and validated as effective assays for detecting neutralizing antibodies against SARS-CoV-2.17,20–22 They can also be used in decentralized areas to estimate the prevalence of COVID-19 antibodies in different groups.
A decline in protective immunity against COVID-19 prompted a third round of vaccination with booster doses worldwide. In Spain, the booster campaign began in January 2022 for the healthcare sector with a third dose of the BNT162b2 (Pfizer-BioNTech) vaccine.23
This study aims to estimate the prevalence and durability of neutralizing antibodies against SARS-CoV-2 induced after a second dose of the BNT162b2 vaccine (Pfizer-BioNTech) in healthcare workers and healthcare support personnel by means of a rapid LFIC-based assay.
MethodsDesign and selection criteriaA descriptive, cross-sectional study was conducted in the Orihuela Health Department (Alicante, Spain). The department comprises 1,500 employees. In Spain, vaccination is each individual’s choice and therefore, participation in our study was voluntary. All healthcare and healthcare support personnel were invited to participate in the study prior to the administration of the booster or third dose of the SARS-CoV-2 vaccine. The study was conducted at the vaccination center during the month of December 2021.
The research team provided all relevant information on the study to each subject and obtained informed consent prior to starting the study procedure. Demographic variables were obtained by means of an anonymous, standardized paper questionnaire distributed prior to the LFIC serological test and prior to vaccination with the booster dose.
Serological testTo evaluate the presence of neutralizing antibodies, the OJABIO® SARS-CoV-2 Neutralizing Antibody Detection Kit (Colloidal 109 Gold Method), manufactured by Wenzhou OJA Biotechnology Co., Ltd. (Wenzhou, Zhejiang, China) was used. It is a point-of-care (POC) antibody detection assay based on the lateral flow immunochromatography (LFIC) technique.
The test was performed according to the manufacturer's recommendations and only using the reagents and materials provided in the kit. The results were interpreted by trained investigators.
The neutralizing antibodies against SARS-CoV-2 present in the sample bind to the SARS-CoV-2 spike protein that is conjugated with a colloidal gold particle. The resulting conjugate migrates toward the reaction matrix attracted by lateral flow. Then, once on the reaction pad, it is captured by anti-immunoglobulin antibodies (IgG or IgM) fixed to the nitrocellulose pad. The binding of the conjugate to the membrane is shown through formation of the test (T) line, which indicates the presence of SARS-CoV-2 neutralizing antibodies. The adjacent control (C) line indicates that the test technique was correctly performed. The presence of two visible lines—C (control) and T (total antibodies)—is considered positive. The presence of the C line alone is interpreted as a negative result. All tests with missing C and T lines were invalid.
This OJABIO® LFIC test was validated in a previous study conducted by the University of Alicante22 against an ELISA (Enzyme-Linked ImmunoSorbent Assay)-based surrogate viral neutralization test (sVNT) in 78 cases and 39 controls, yielding a specificity for the OJABIO® test of 1.00 (95% CI: 0.91–1.00) and a sensitivity of 0.99 (95% CI: 0.93–1.00).
Variables and statistical analysisThe demographic variables collected were age (in years) and sex. In addition, the following epidemiological and clinical variables were collected: previous SARS-CoV-2 infection (defined as a positive PCR test), time since last dose (TLD) (defined as weeks since vaccination, the last dose before the study test, or a positive PCR test for SARS-CoV-2 before the study test), having a chronic disease, having overcome a recent infection (in the last month), and vaccines administered with the dates and number of previous doses. Statistical analysis was performed by calculating the odds ratio (OR) for each qualitative variable. Logistic regression was used to calculate the OR for quantitative variables. For age, ten-year periods were used. To calculate the adjusted ORs (aOR), a multivariate stepwise logistic regression model was performed using all the variables mentioned above. The 95% confidence intervals were calculated. Statistical significance was defined as a p value less than 0.05. The data were analyzed using SPSS 23.0 statistical software.
Ethical considerationsThe study was conducted in accordance with the Declaration of Helsinki regarding clinical trials in human beings. The research proposal was approved by the Research Ethics Committee of the University of Alicante (Spain) (file UA-2021-05-07_5, dated 05/24/2021) and the Clinical Research Ethics Committee of the Orihuela Health Department (Spain) (MC-2021-023, dated 11/15/2021).
ResultsThe Orihuela Health Department comprises 1,500 healthcare and healthcare support personnel. Of them, 1,352 participated in the study and 977 responded to the survey. Among the survey respondents, 13 subjects were excluded because their epidemiological survey was incomplete. The contact rate was 90.1% (1,352/1,500), the response rate was 72.3% (977/1,352, and the eligibility rate was 98.7% (964/977).24
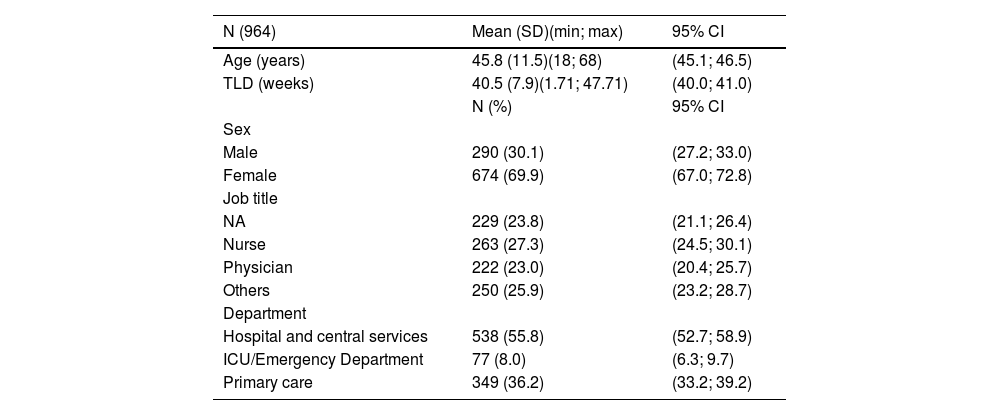
Of the total sample, 674 (69.9%) were women (the male:female ratio was 0.43) and the mean age (± SD) was 45.8 ± 11.5 years. A total of 23.8% were nurse aides (NA), 27.3% were nurses, and 23.0% were physicians. Over 55% were part of hospital ward units and central services.
Table 1 shows the distribution of the population according to demographics and position.
Socio-demographic characteristics.
| N (964) | Mean (SD)(min; max) | 95% CI |
|---|---|---|
| Age (years) | 45.8 (11.5)(18; 68) | (45.1; 46.5) |
| TLD (weeks) | 40.5 (7.9)(1.71; 47.71) | (40.0; 41.0) |
| N (%) | 95% CI | |
| Sex | ||
| Male | 290 (30.1) | (27.2; 33.0) |
| Female | 674 (69.9) | (67.0; 72.8) |
| Job title | ||
| NA | 229 (23.8) | (21.1; 26.4) |
| Nurse | 263 (27.3) | (24.5; 30.1) |
| Physician | 222 (23.0) | (20.4; 25.7) |
| Others | 250 (25.9) | (23.2; 28.7) |
| Department | ||
| Hospital and central services | 538 (55.8) | (52.7; 58.9) |
| ICU/Emergency Department | 77 (8.0) | (6.3; 9.7) |
| Primary care | 349 (36.2) | (33.2; 39.2) |
SD: Standard Deviation; min: minimum; max: maximum; ICU: Intensive Care Unit; TLD: Time since last dose; CI: Confidence Interval; NA: Nurse aide.
The BioNTech/Pfizer® vaccine was administered to 97.5% (940/964) of all subjects. Two doses had been administered in 98.5% (950/964) and 86.4% (833/964) had not been diagnosed with previous COVID-19 infection.
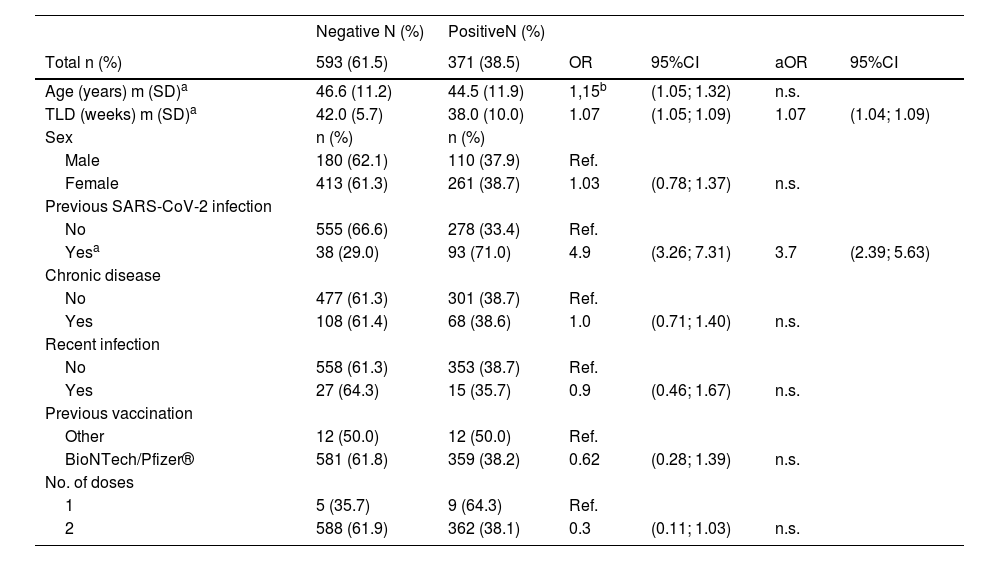
In total, 964 subjects were tested for SARS-CoV-2 neutralizing antibodies using the OJABIO® SARS-CoV-2 neutralizing antibody detection kit. Of them, 593 (61.5%) were negative and 371 (38.5%) were positive. On the multivariate analysis, the time since the last vaccine dose (aOR week: 1.07; 95%CI: 1.04; 1.09) and previous SARS-CoV-2 infection (aOR: 3.7; 95%CI: 2.39; 5.63) were statistically significant in regard to a positive serological result (Table 2). Specifically, the probability of a positive result in subjects who had had COVID-19 infection was approximately four times higher than in those who had not.
Serological test results with respect to demographic and epidemiological-clinical variables.
| Negative N (%) | PositiveN (%) | |||||
|---|---|---|---|---|---|---|
| Total n (%) | 593 (61.5) | 371 (38.5) | OR | 95%CI | aOR | 95%CI |
| Age (years) m (SD)a | 46.6 (11.2) | 44.5 (11.9) | 1,15b | (1.05; 1.32) | n.s. | |
| TLD (weeks) m (SD)a | 42.0 (5.7) | 38.0 (10.0) | 1.07 | (1.05; 1.09) | 1.07 | (1.04; 1.09) |
| Sex | n (%) | n (%) | ||||
| Male | 180 (62.1) | 110 (37.9) | Ref. | |||
| Female | 413 (61.3) | 261 (38.7) | 1.03 | (0.78; 1.37) | n.s. | |
| Previous SARS-CoV-2 infection | ||||||
| No | 555 (66.6) | 278 (33.4) | Ref. | |||
| Yesa | 38 (29.0) | 93 (71.0) | 4.9 | (3.26; 7.31) | 3.7 | (2.39; 5.63) |
| Chronic disease | ||||||
| No | 477 (61.3) | 301 (38.7) | Ref. | |||
| Yes | 108 (61.4) | 68 (38.6) | 1.0 | (0.71; 1.40) | n.s. | |
| Recent infection | ||||||
| No | 558 (61.3) | 353 (38.7) | Ref. | |||
| Yes | 27 (64.3) | 15 (35.7) | 0.9 | (0.46; 1.67) | n.s. | |
| Previous vaccination | ||||||
| Other | 12 (50.0) | 12 (50.0) | Ref. | |||
| BioNTech/Pfizer® | 581 (61.8) | 359 (38.2) | 0.62 | (0.28; 1.39) | n.s. | |
| No. of doses | ||||||
| 1 | 5 (35.7) | 9 (64.3) | Ref. | |||
| 2 | 588 (61.9) | 362 (38.1) | 0.3 | (0.11; 1.03) | n.s. |
Regarding age, there were significant differences when it was considered an independent variable; it was found that the older the age, the lower the positivity rate (OR 10 years): 1.15; 95% CI: 1.05–1.32). However, on the multivariate analysis, this variable was not found to be statistically significant.
It was observed that the time since last dose (TLD) variable was statistically significant and inversely correlated to the probability of a positive serological test.
The variables of sex, presence of chronic disease, recent infection, type of previous vaccines, and number of doses showed no statistically significant differences.
DiscussionIn the Orihuela Health Department, 38.5% of healthcare workers tested positive for neutralizing antibodies against SARS-CoV-2 prior to administration of the booster dose. Similar studies have warned of a decrease in immunity against SARS-CoV-2 infection in all age groups a few months after receiving the second dose of BNT162b2 vaccine.8–10,12,13 Specifically, in a prospective study on healthcare personnel, the level of neutralizing antibodies declined rapidly during the first three months and at a relatively slower pace thereafter. The decline was more prominent in men, those aged 65 years or older, and immunosuppressed individuals.8 In this study, there were no statistically significant differences in a positive test result according to age. In contrast, age seems to influence the probability of a positive result for SARS-CoV-2 neutralizing antibodies.
Another factor that was observed to have an influence on a positive serological test result was the time since the last dose was administered. Subjects who had not received a dose for a longer period of time showed a lower rate of positive results for neutralizing antibodies against SARS-CoV-2. In this study, the time since the last vaccine dose was quite long (40.5 weeks or around ten months). This could have had an important impact on the serological deterioration associated with the presence of SARS-CoV-2 neutralizing antibodies. In addition, it may also be associated with a higher risk of infection since breakthrough infections increase significantly as more time passes since the administration of the second BNT162b2 dose10 as well as with a lower titer of neutralizing antibodies against SARS-CoV-2.25
On the other hand, these results reflect a significant association between previous SARS-CoV-2 infection and a positive serological result for neutralizing antibodies against SARS-CoV-2. These data are similar to those reported in a recently published study conducted at the University of Alicante, which observed a strong association between both variables.26 Furthermore, this association has been observed in other studies in this setting. One such study found that the levels of SARS-CoV-2 antibodies against the S or “spike” protein in healthcare workers with previous COVID-19 infection six months after vaccination were higher than in those who were vaccinated but without a history of COVID-19,27 with a higher mean SARS-CoV-2 antibody titer and slower serological deterioration.28,29 Furthermore, it found an 84% lower risk of infection, with the median protective effect observed seven months after the primary infection.30
To mitigate this decrease in vaccine protection, a third booster dose of the BNT162b2 SARS-CoV-2 vaccine was evaluated in clinical trials.9,25 Results obtained from real-world studies have shown that a third dose of the BNT162b2 mRNA vaccine is effective for protecting individuals against severe cases of COVID-19, concluding that although the vaccine is not able to prevent infection, it is able to reduce severity.31–33 For this reason, the relevant health authorities provided for the administration of a third vaccine dose in at-risk groups, including healthcare personnel, in their vaccination strategies.23 All study participants received the third vaccine dose the day after the study, in accordance with health authorities’ instructions.
The test used in this study was an LFIC assay, which allows for obtaining immediate results. This serological test was validated for the detection of neutralizing antibodies against SARS-CoV-2 in mid-2021.22 This study was conducted in December 2021, so the appearance of emerging variants in the time between test validation and the study could be a limitation. However, the BNT162b2 vaccine has been shown to be effective for all variants prior to the omicron variant,34 which was not predominant in Spain until January 2022.35
In the case of vaccination against COVID-19, the procedure is similar to that of influenza vaccination: the vaccine’s composition is updated when a greater than four-fold reduction in the neutralizing activity against new circulating virus strains compared to previous strains is observed.36 In fact, as of September 2023, the latest version of the Comirnaty BioNtech/Pfizer vaccine encodes the SARS-CoV-2 virus spike (S) protein from the Omicron XBB.1.5 strain instead of the original Wuhan strain.37
ConclusionThe data suggest that the duration of SARS-CoV-2 neutralizing antibody protection is strongly influenced by the time elapsed since the administration of the last vaccine dose and by having had COVID-19 infection prior to the booster dose.
FundingThis work was supported by a consultancy contract from the University of Alicante with reference WENZHOU-OJABIO1-21T, which made the rapid serological tests provided by the OJABIO company available. No authors received personal remuneration from the biotechnology company.
Conflicts of interestThe authors declare that they do not have any conflicts of interest. The funding entities had no role in the study design; in data collection, analysis, or interpretation; in the writing of the manuscript; or in the decision to publish the results.
We would like to thank all the health workers who participated in this study. In particular, we would like to thank the director of the Orihuela Health Department, Dr. Miguel Elías Fayos, for his support. We are also grateful to the Nursing Department Supervisors at the Health Department for their excellent work.