Currently, the usefulness of lung ultrasound in the follow-up of patients after hospital discharge for SARS-CoV-2 pneumonia is not well known. The main objective of this systematic review is to investigate the persistence of alterations in lung ultrasound of patients who have had COVID-19 pneumonia.
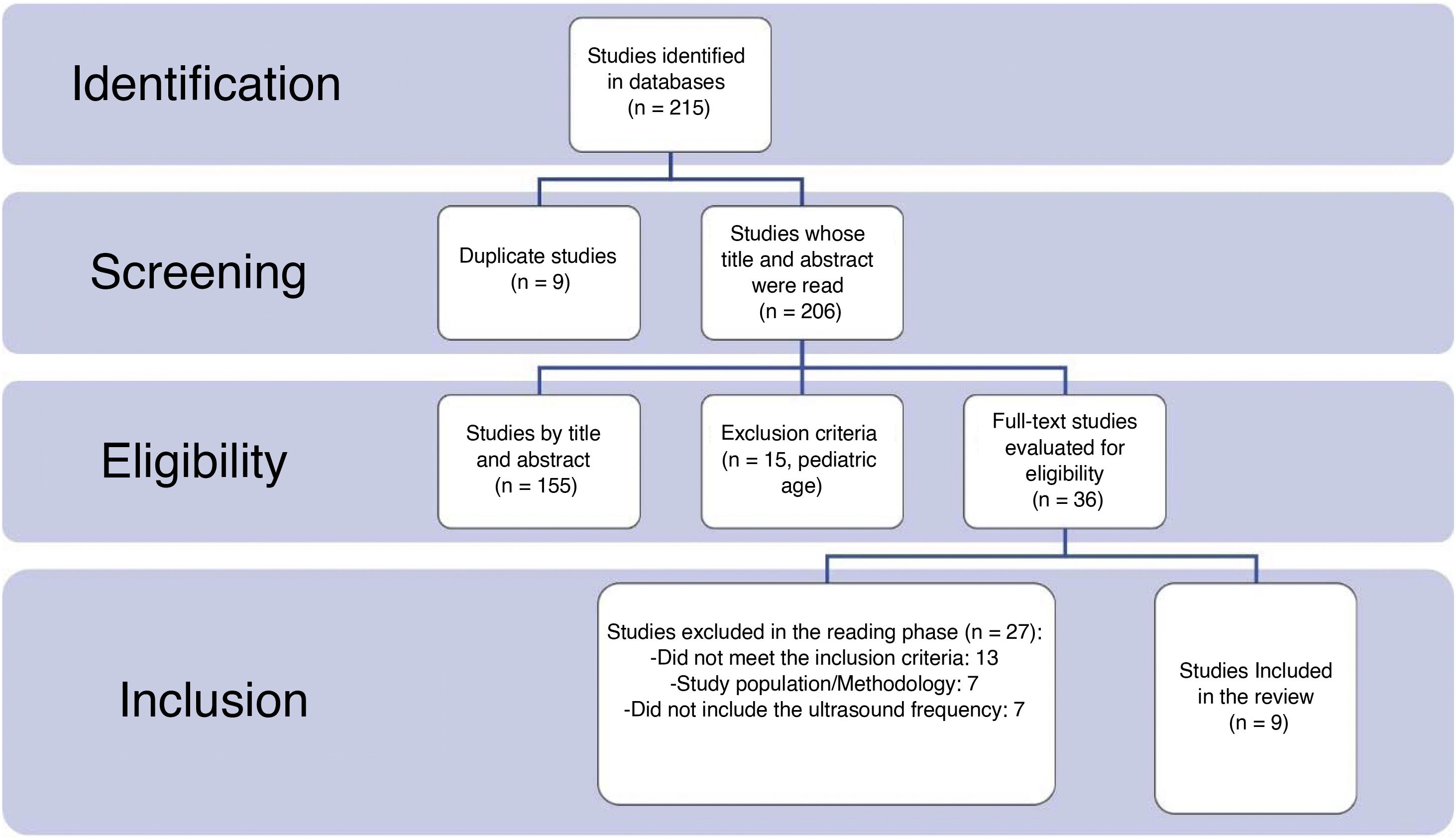
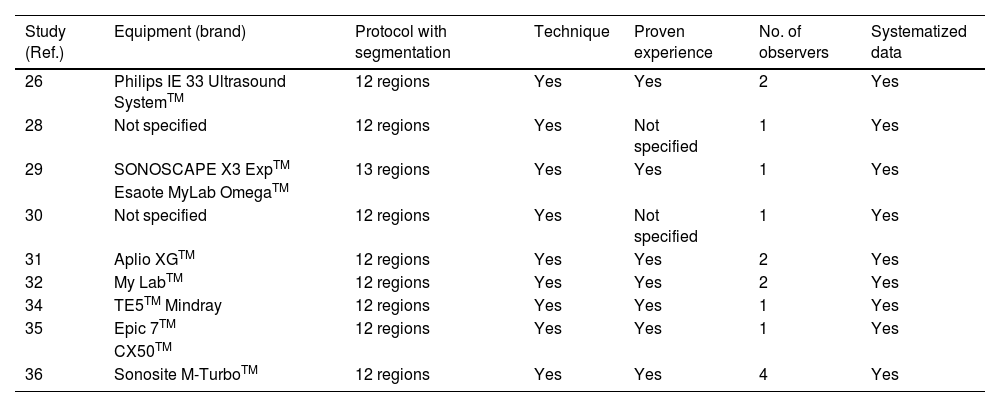
MethodsA systematic review has been carried out following the PRISMA regulations in the PubMed, EMBASE, Web of Science and Google Scholar database from January 2020 to May 2023 using the combination of MeSH terms: “lung ultrasound”, “ultrasonography”, “lung alterations”, “persistence”, “follow-up”, “consequences”, “hospital discharge”, “COVID”, “COVID-19”, “SARS-CoV-2”. Studies were selected that described alterations in the lung ultrasound of patients after having suffered from COVID-19 pneumonia. The JBI Critical Appraisal Tools were used to assess the risk of bias of the studies. No meta-analysis techniques were performed, the results being compared narratively.
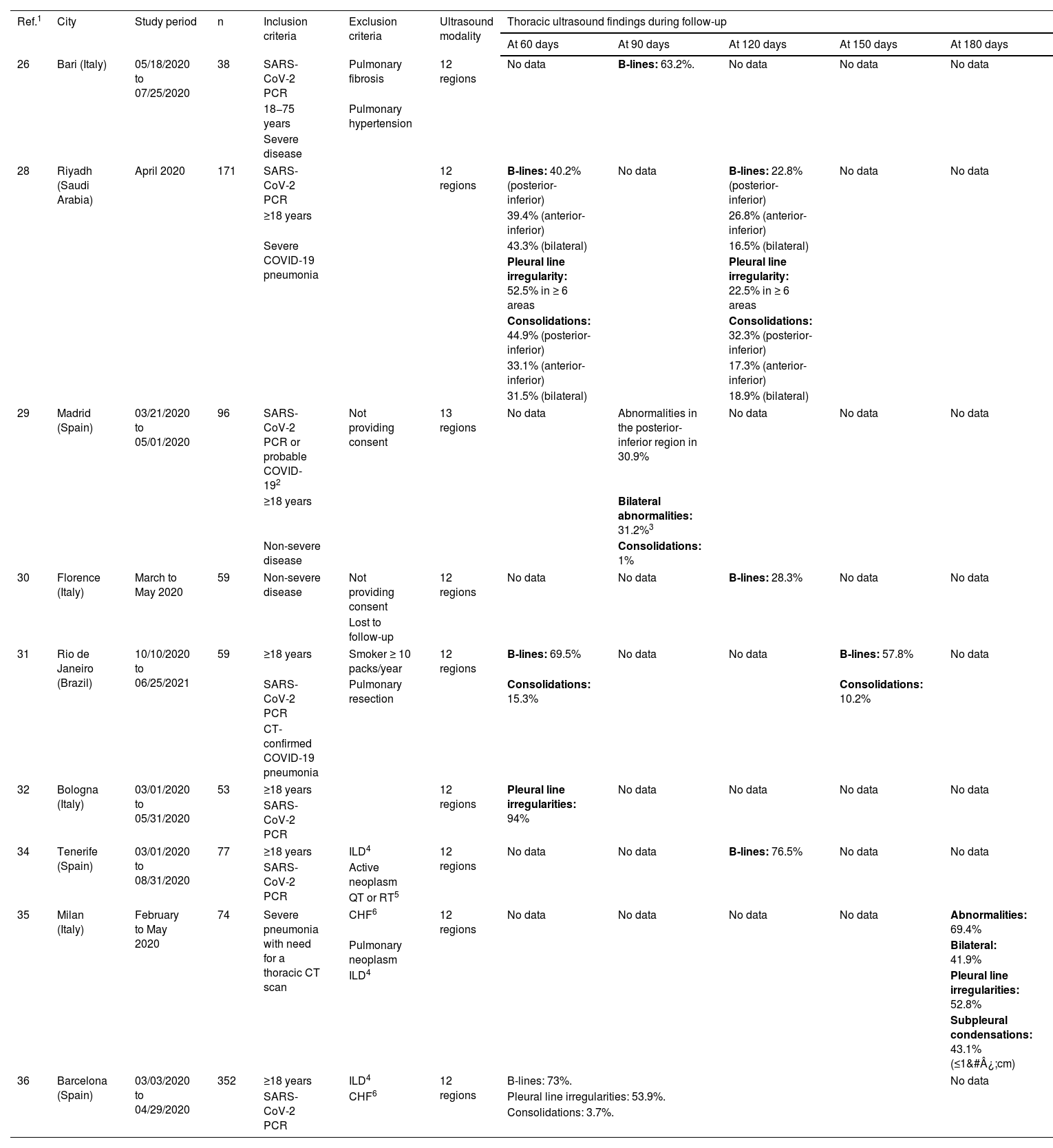
ResultsFrom two to six months after COVID-19 pneumonia, pulmonary ultrasound abnormalities appear frequently and are proportional to the intensity of the initial episode. The most frequent anomalies are irregularities in the pleural line, the presence of B lines and/or subpleural consolidations, predominantly in the basal regions of the thorax. These findings seem to correlate with those of the chest CT.
ConclusionsLung ultrasound offers technical and economic advantages that should be considered for the study of patients after hospital discharge for COVID-19.
Actualmente no se conoce bien la utilidad de la ecografía pulmonar en el seguimiento de los pacientes tras haber tenido una hospitalización por neumonía por SARS-CoV-2. El objetivo principal de esta revisión sistemática es investigar la persistencia de las alteraciones en la ecografía pulmonar de los pacientes que han tenido una neumonía COVID-19.
MétodosSe ha realizado una revisión sistemática siguiendo la normativa PRISMA en la base de datos PubMed, EMBASE, Web os Science y Google Scholar desde enero del 2020 hasta mayo del 2023 utilizando la combinación de términos MeSH: lung ultrasound, ultrasonography, lung alterations, persistence, follow-up, consequences, hospital discharge, COVID, COVID-19, SARS-CoV-2. Se seleccionaron estudios que describieran alteraciones en la ecografía pulmonar de pacientes tras haber padecido una neumonía COVID-19. Se empleó la JBI Critical Appraisal Tools para evaluar el riesgo de sesgos de los estudios. No se realizaron técnicas de metaanálisis, siendo los resultados comparados narrativamente.
ResultadosDe dos a seis meses después de la neumonía COVID-19, las alteraciones ecográficas pulmonares aparecen con frecuencia y son proporcionales a la intensidad del episodio inicial. Las anomalías más frecuentes son las irregularidades de la línea pleural, la presencia de líneas B y/o consolidaciones subpleurales, con predominio por las regiones basales del tórax. Estos hallazgos parecen correlacionarse con los de TC de tórax.
ConclusionesLa ecografía pulmonar ofrece unas ventajas técnicas y económicas que deben considerarse para el estudio de los pacientes tras el alta hospitalaria por COVID-19.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<