Determine the incidence and risk factors of type 2 diabetes in the adult population of Madrid (Spain) and compare the predictive models of type 2 diabetes based on the prediabetes criteria of the American Diabetes Association (ADA) and the World Health Organisation (WHO).
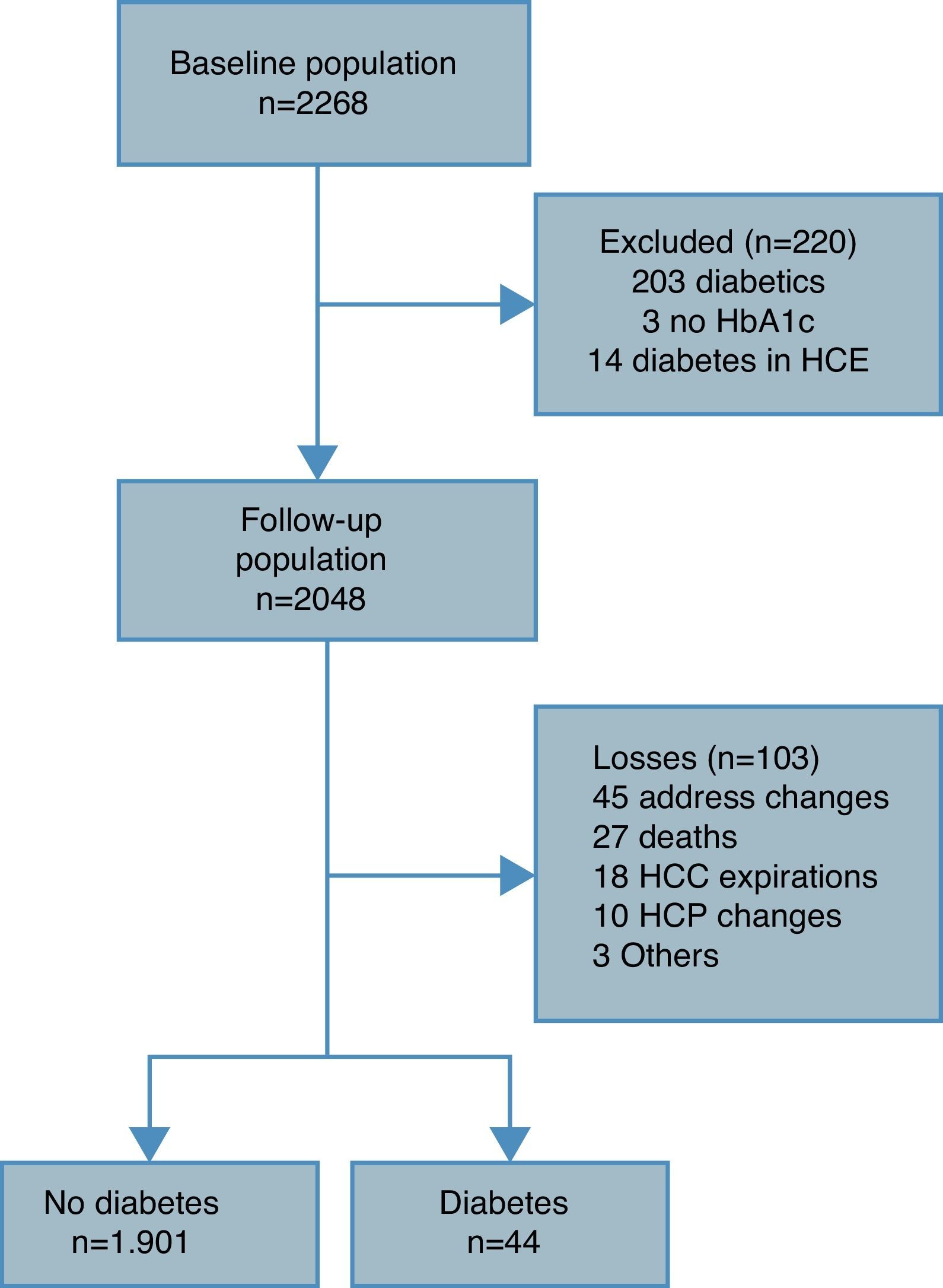
Material and methodsA prospective study was conducted on a population cohort composed of 2048 individuals between 30 and 74years of age with no diabetes. At the start of the study, an epidemiological survey was performed, and baseline glycaemia, HbA1c, body mass index and waist circumference were measured. A follow-up of 6.4years was conducted. New cases of type 2 diabetes were identified using the electronic primary care medical history.
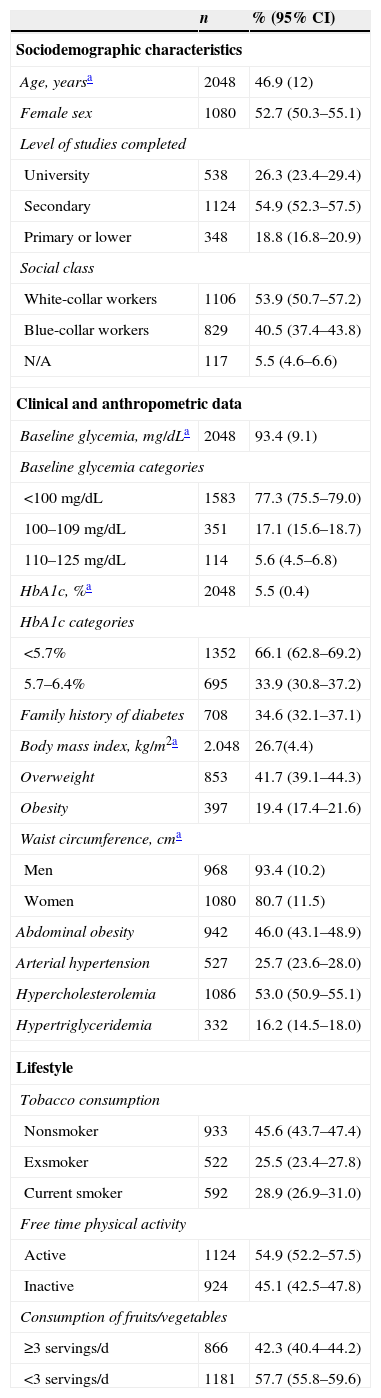
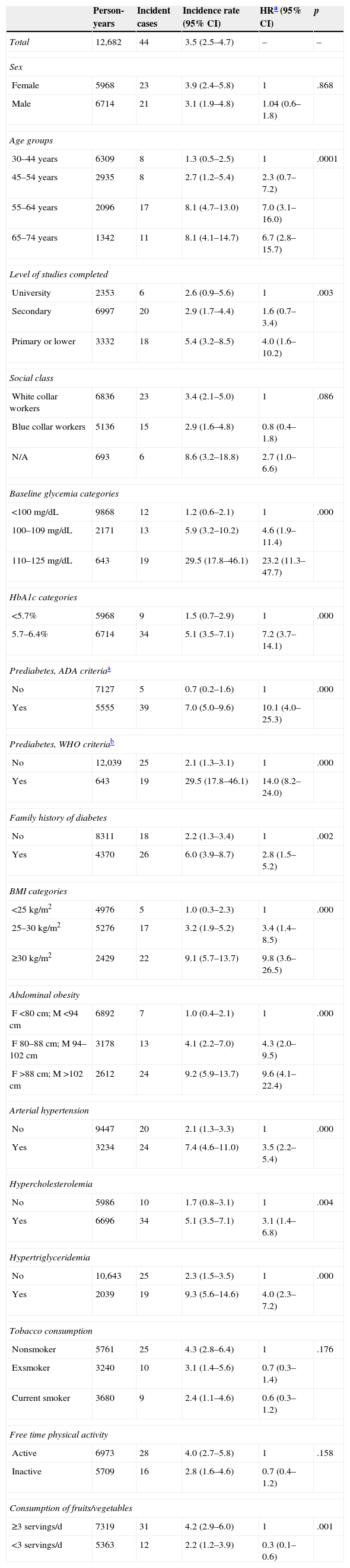
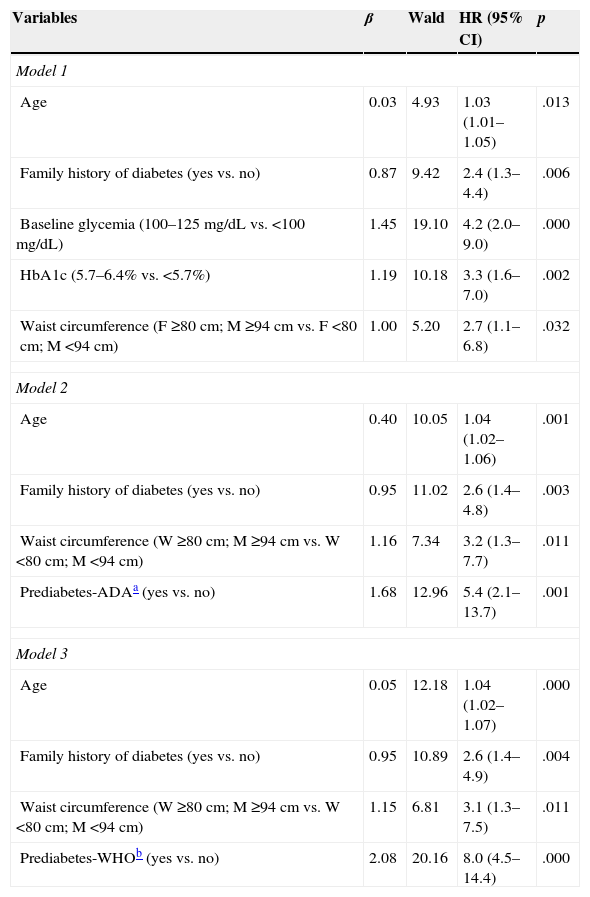
ResultsThe incidence of type 2 diabetes was 3.5 cases/1000 person-years. In the multivariate analysis, the variables that were associated with the onset of type 2 diabetes were age, family history of diabetes, baseline glycaemia (100–125mg/dL), HbA1c (5.7–6.4%) and waist circumference (≥94cm for men and ≥80cm for women). Of these, the most significantly associated variables were baseline glycaemia and HbA1c. The ADA and WHO criteria for defining prediabetes had the same predictive capacity.
ConclusionThe incidence of type 2 diabetes measured in Madrid was lower than that found in other population studies, with the glucometabolic state the main factor associated with progression to type 2 diabetes. There were no differences between the prediabetes defined by the ADA and the WHO for predicting the onset of the disease.
Estimar la incidencia y los factores de riesgo de diabetes tipo 2 en la población adulta de Madrid (España) y comparar los modelos predictivos de diabetes tipo 2 basados en los criterios de prediabetes de la American Diabetes Association (ADA) y la Organización Mundial de la Salud (OMS).
Material y métodosEstudio prospectivo de una cohorte poblacional formada por 2048 individuos de entre 30 y 74años sin diabetes. Al inicio del estudio se realizó una encuesta epidemiológica y se midió la glucemia basal, la HbA1c, el índice de masa corporal y el perímetro de la cintura. Se realizó un seguimiento de 6,4años. Los casos nuevos de diabetes tipo 2 se identificaron a través de la historia clínica electrónica de atención primaria.
ResultadosLa incidencia de diabetes tipo 2 fue 3,5 casos/1.000 personas-año. En el análisis multivariante las variables que se asociaron con la aparición de diabetes tipo 2 fueron la edad, los antecedentes familiares de diabetes, la glucemia basal (100–125mg/dL), la HbA1c (5,7–6,4%) y el perímetro de la cintura (≥94cm en hombres y ≥80cm en mujeres). De estas, las más significativamente asociadas fueron la glucemia basal y la HbA1c. Los criterios de la ADA y la OMS para definir prediabetes tuvieron la misma capacidad predictiva.
ConclusiónLa incidencia de diabetes tipo 2 estimada en Madrid fue inferior a la encontrada en otros estudios poblacionales, siendo el estado glucometabólico el principal factor asociado a la progresión a diabetes tipo 2. No se han detectado diferencias entre la prediabetes definida por la ADA y la OMS para predecir la aparición de la enfermedad.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<