To describe clinical features, comorbidity, and prognostic factors associated with in-hospital mortality in a cohort of COVID-19 admitted to a general hospital.
Material and methodsRetrospective cohort study of patients with COVID-19 admitted from 26th February 2020, who had been discharged or died up to 29th April 2020. A descriptive study and an analysis of factors associated with intrahospital mortality were performed.
ResultsOut of the 101 patients, 96 were analysed. Of these, 79 (82%) recovered and were discharged, and 17 (18%) died in the hospital. Diagnosis of COVID-19 was confirmed by polymerase chain reaction to SARS-CoV2 in 92 (92.5%). The mean age was 63 years, and 66% were male. The most frequent comorbidities were hypertension (40%), diabetes mellitus (16%) y cardiopathy (14%). Patients who died were older (mean 77 vs 60 years), had higher prevalence of hypertension (71% vs 33%), and cardiopathy (47% vs 6%), and higher levels of lactate dehydrogenase (LDH) and reactive C protein (mean 662 vs 335 UI/L, and 193 vs 121mg/L respectively) on admission. In a multivariant analysis the variables significantly associated to mortality were the presence of cardiopathy (CI 95% OR 2,58–67,07), levels of LDH≥345 IU/L (CI 95% OR 1,52–46,00), and age≥65 years (CI 95% OR 1,23–44,62).
ConclusionsThe presence of cardiopathy, levels of LDH≥345 IU/L and age≥65 years, are associated with a higher risk of death during hospital stay for COVID-19. This model should be validated in prospective cohorts.
Describir el perfil clínico, comorbilidad y factores pronósticos de mortalidad intrahospitalaria en una cohorte COVID-19 de un hospital general.
Material y métodosEstudio de cohortes retrospectivo de pacientes con COVID-19 ingresados desde el 26 de febrero de 2020, y dados de alta o fallecidos hasta el 29 de abril; estudio descriptivo y análisis de factores asociados a la mortalidad intrahospitalaria.
ResultadosDe los pacientes ingresados (N=101), fueron analizados 96, siendo dados de alta por curación 79 (82%), y falleciendo 17 (18%). Se confirmó COVID-19 por reacción en cadena de la polimerasa a SARS-CoV-2 en 92 casos (92,5%). La edad media fue de 63 años y 66% fueron varones. La comorbilidad previa más frecuente fue hipertensión arterial (40%), diabetes mellitus (16%) y cardiopatía (14%). Los pacientes que fallecieron tenían significativamente más edad (media 77 vs. 60 años), hipertensión arterial (71% vs 33%), cardiopatía previa (47% vs. 6%), y niveles más elevados de lactato deshidrogenasa (LDH) (662 vs. 335 UI/L) y proteína C reactiva (PCR) (193 vs. 121mg/L) al ingreso. En análisis multivariante, se asociaron significativamente a mayor riesgo de muerte la presencia de cardiopatía (IC 95% OR 2,58–67,07), los niveles de LDH≥345 UI/L (IC 95% OR 1,52–46,00), y la edad≥65 años (IC 95% OR 1,23–44,62).
ConclusionesEl antecedente de cardiopatía, niveles de LDH≥345 UI/L al ingreso, y una edad≥65 años, se asocian a mayor mortalidad durante el ingreso por COVID-19. Hay que validar este modelo pronóstico en cohortes prospectivas.
In December 2019, the first cases of pneumonia originating from a new strain of coronavirus (SARS-CoV-2) appeared in Wuhan (China). The new disease was named COVID-19. On January 31, 2020, the first imported case was recorded in Spain and on February 26, the first local transmission was registered. On March 11, the World Health Organization declared a pandemic.
As of May 10, 2020, a total of 3,986,119 cases had been reported worldwide, with 278,814 deaths.1 At that time, Spain was the second country in the world in terms of most reported cases: 224,390 cases confirmed by SARS-CoV-2 polymerase chain reaction (SARS-CoV-2 PCR), with 26,621 deaths and 136,166 infections resolved.2
Since the start of the pandemic, treatment recommendations based on studies with many limitations in terms of size and design have been incorporated into clinical practice. There are still no specific treatments available that have a solid scientific backing. Treatment of patients is based on symptomatic treatment, especially oxygen, and on pharmaceutical treatment protocols, with the recommendation of including patients in clinical trials where possible.
In the absence of effective treatment, knowledge of patient characteristics that can predict poor progress at the time of admission can be useful for identifying those who should be hospitalized or those for whom support measures should be intensified or recommended treatments started early. In this study, we describe the first cases of COVID-19 in patients hospitalized in a general hospital and analyze the characteristics upon admission associated with in-hospital death.
Materials and methodsA retrospective cohort study was performed on consecutive patients hospitalized due to COVID-19 in the Costa del Sol Hospital in Marbella (Málaga, Spain). Patients were included starting with the first case admitted on February 26, 2020 up until the end of the study period on April 29, 2020. They were either discharged after recovery or died in the hospital. Diagnosis was confirmed by a positive SARS-CoV-2 PCR test on nasopharyngeal, sputum, or bronchoaspiration samples and/or a positive serology and compatible clinical symptoms.
A nasopharyngeal and sputum sample was obtained when the patient was able to expectorate. If the SARS-CoV-2 PCR test of both samples was negative and the clinical suspicion persisted, the possibility of taking another set of samples and/or performing a bronchoscopy and taking lower respiratory specimens was evaluated. The presence of SARS-CoV-2 was detected in respiratory samples by means of reverse transcription-polymerase chain reaction (RT-PCR) from 2 suppliers (VIASURE SARS-CoV-2 from CerTest Biotec and LightMix Modular SARS-CoV (COVID-19) from Roche). Samples with a cycle threshold (Ct) between 38 and 40 were repeated using another RT-PCR technique. IgG and IgM antibodies were detected in serum by means of immunochromatography in the solid phase (COVID-19 IgG/IgM Rapid Test Cassette, Zhejiang Orient Gene Biotech Co. Ltd.).
The Costa del Sol Hospital is a 387-bed specialty hospital. Its reference population encompasses 410,022 residents. There are 12 intensive care unit (ICU) beds, which were temporarily increased to 31 by expanding to other hospital areas.
There was no protocol for collecting informed consent given that the information used was gathered solely from the medical record. Both obtaining a patient’s signature and then filing it were considered unnecessary risks given that the population had an infectious disease, in addition to the fact that the results are considered to be in the interest of public health. All the data collected were recorded anonymously, in strict accordance with current data protection laws and regulations (Organic Law 3/2018, of December 5, on Personal Data Protection and the Guarantee of Digital Rights).
Since the first admission for COVID-19 on February 26, 2020, all data on hospital admissions due to COVID-19 have been included in a prospective database that includes demographic variables, medical history, and clinical and analytical data upon admission. During the hospital stay, clinical, radiological, and analytical data on the patients’ progress were recorded.
Among the baseline characteristics recorded, the following were included: age, sex, comorbidity, tobacco use, use of angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARB), and polypharmacy (defined as use of more than 5 drugs). Variables on the initial clinical presentation (symptoms, baseline SatO2, analytical and radiological findings) were recorded. We carried out a descriptive analysis with quantitative variables expressed as means and standard deviation (SD) and qualitative variables expressed as absolute numbers and percentages.
A bivariate analysis was performed to detect differences between survivors and non-survivors. Student’s t-test was used for continuous variables and the hi-square or Fisher’s exact test for categorical variables. A multivariate logistical regression model was created using in-hospital death as the outcome variable and including variables that were found to be statistically significant on the raw analysis (quantitative variables were dichotomized at the median in order to facilitate interpretation of the model). P<0.05 was used as the entry criterion and p>0.1 as the exit criterion, with the forward selection method used according to likelihood. Variance was evaluated through Nagelkerke R2 and goodness of fit using the Hosmer–Lemeshow test, describing the odds ratio with its respective 95% confidence intervals. On the statistical analyses, the level of significance was established as p<0.05. The SPSS program version 15 was used for the statistical analyses.
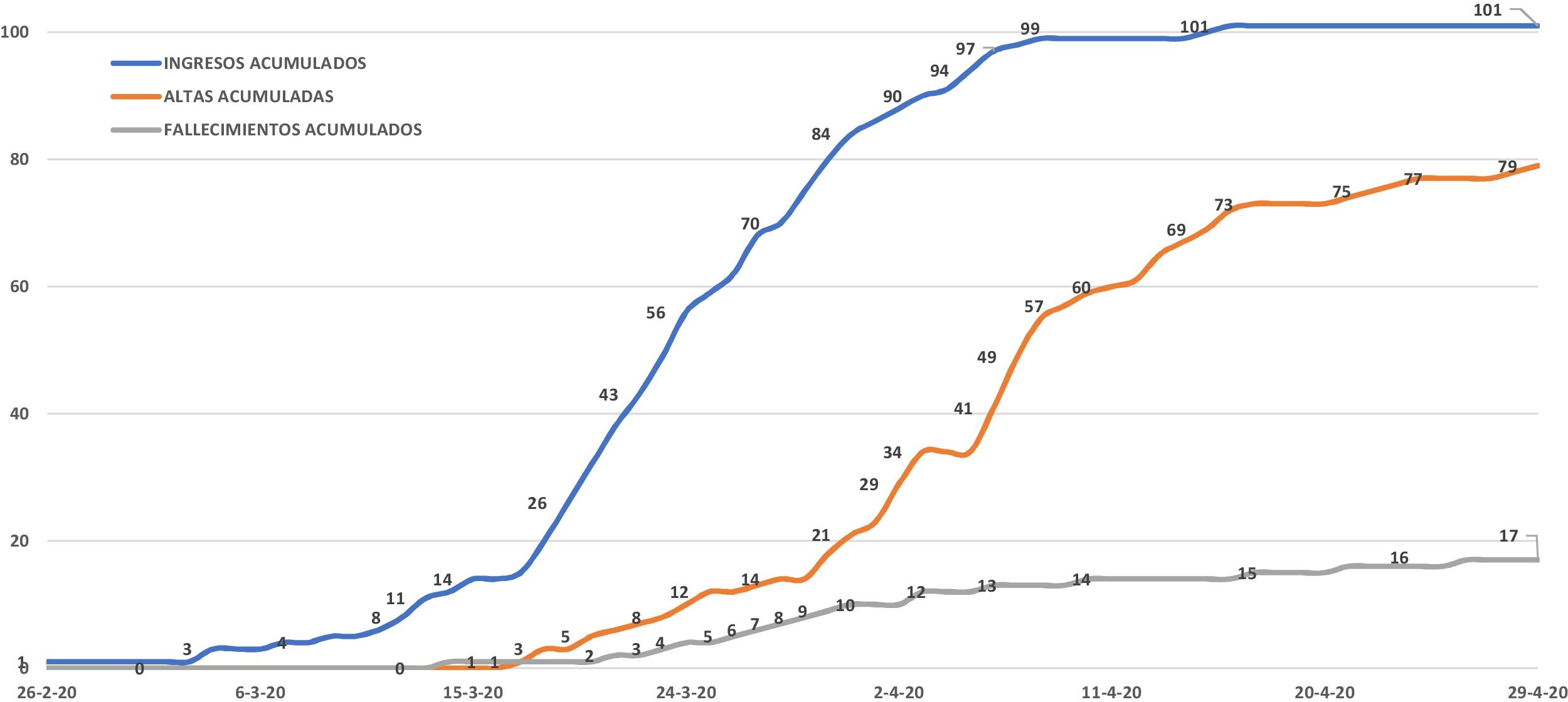
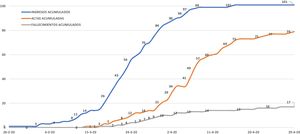
ResultsDuring the study period, 101 patients were admitted due to COVID-19 at the Costa del Sol Hospital. As of April 29, 96 had been discharged or died; these patients were the study group. The other five patients were excluded as they remained hospitalized (Fig. 1). At the end of the study period, COVID-19 cases in the hospital's coverage area had risen to 395, with 21 deaths (incident rate of 96.34 and mortality rate of 5.12 per 100,000 inhabitants, respectively). The case fatality rate was 5.31%.
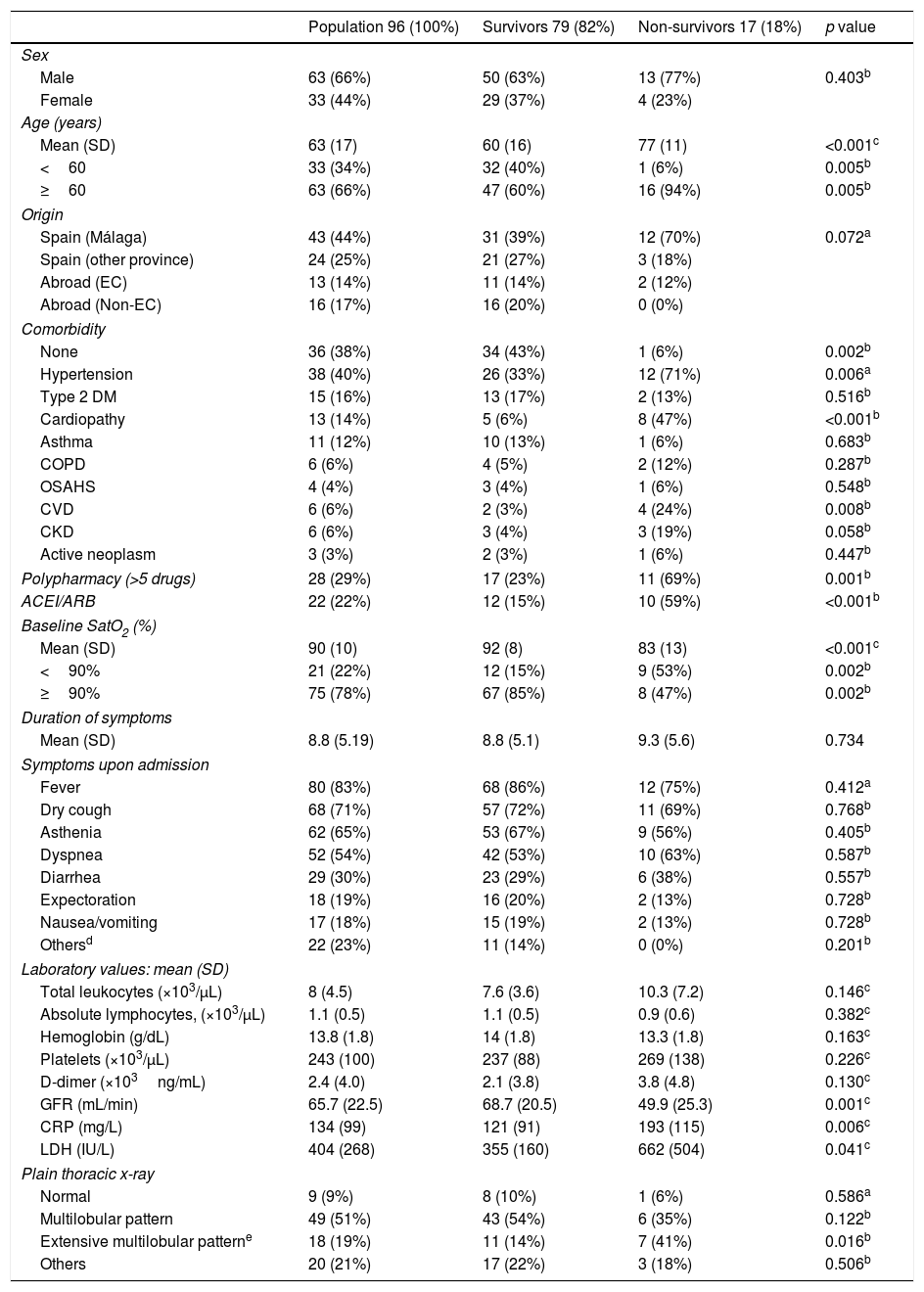
In Table 1, patients’ baseline characteristics and clinical, radiological, and laboratory findings upon admission are shown. The mean age was 63 years and 66% were male. A total of 38% of patients presented with no comorbidity and 60% had no toxic habits. The most frequent comorbidities were hypertension, diabetes mellitus, and cardiopathy. Nearly one quarter of patients were in treatment with ACEIs or ARBs and 29% had polypharmacy.
Baseline characteristics upon admission analyzed by subgroups according to clinical progress: survivors and non-survivors.
| Population 96 (100%) | Survivors 79 (82%) | Non-survivors 17 (18%) | p value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 63 (66%) | 50 (63%) | 13 (77%) | 0.403b |
| Female | 33 (44%) | 29 (37%) | 4 (23%) | |
| Age (years) | ||||
| Mean (SD) | 63 (17) | 60 (16) | 77 (11) | <0.001c |
| <60 | 33 (34%) | 32 (40%) | 1 (6%) | 0.005b |
| ≥60 | 63 (66%) | 47 (60%) | 16 (94%) | 0.005b |
| Origin | ||||
| Spain (Málaga) | 43 (44%) | 31 (39%) | 12 (70%) | 0.072a |
| Spain (other province) | 24 (25%) | 21 (27%) | 3 (18%) | |
| Abroad (EC) | 13 (14%) | 11 (14%) | 2 (12%) | |
| Abroad (Non-EC) | 16 (17%) | 16 (20%) | 0 (0%) | |
| Comorbidity | ||||
| None | 36 (38%) | 34 (43%) | 1 (6%) | 0.002b |
| Hypertension | 38 (40%) | 26 (33%) | 12 (71%) | 0.006a |
| Type 2 DM | 15 (16%) | 13 (17%) | 2 (13%) | 0.516b |
| Cardiopathy | 13 (14%) | 5 (6%) | 8 (47%) | <0.001b |
| Asthma | 11 (12%) | 10 (13%) | 1 (6%) | 0.683b |
| COPD | 6 (6%) | 4 (5%) | 2 (12%) | 0.287b |
| OSAHS | 4 (4%) | 3 (4%) | 1 (6%) | 0.548b |
| CVD | 6 (6%) | 2 (3%) | 4 (24%) | 0.008b |
| CKD | 6 (6%) | 3 (4%) | 3 (19%) | 0.058b |
| Active neoplasm | 3 (3%) | 2 (3%) | 1 (6%) | 0.447b |
| Polypharmacy (>5 drugs) | 28 (29%) | 17 (23%) | 11 (69%) | 0.001b |
| ACEI/ARB | 22 (22%) | 12 (15%) | 10 (59%) | <0.001b |
| Baseline SatO2 (%) | ||||
| Mean (SD) | 90 (10) | 92 (8) | 83 (13) | <0.001c |
| <90% | 21 (22%) | 12 (15%) | 9 (53%) | 0.002b |
| ≥90% | 75 (78%) | 67 (85%) | 8 (47%) | 0.002b |
| Duration of symptoms | ||||
| Mean (SD) | 8.8 (5.19) | 8.8 (5.1) | 9.3 (5.6) | 0.734 |
| Symptoms upon admission | ||||
| Fever | 80 (83%) | 68 (86%) | 12 (75%) | 0.412a |
| Dry cough | 68 (71%) | 57 (72%) | 11 (69%) | 0.768b |
| Asthenia | 62 (65%) | 53 (67%) | 9 (56%) | 0.405b |
| Dyspnea | 52 (54%) | 42 (53%) | 10 (63%) | 0.587b |
| Diarrhea | 29 (30%) | 23 (29%) | 6 (38%) | 0.557b |
| Expectoration | 18 (19%) | 16 (20%) | 2 (13%) | 0.728b |
| Nausea/vomiting | 17 (18%) | 15 (19%) | 2 (13%) | 0.728b |
| Othersd | 22 (23%) | 11 (14%) | 0 (0%) | 0.201b |
| Laboratory values: mean (SD) | ||||
| Total leukocytes (×103/μL) | 8 (4.5) | 7.6 (3.6) | 10.3 (7.2) | 0.146c |
| Absolute lymphocytes, (×103/μL) | 1.1 (0.5) | 1.1 (0.5) | 0.9 (0.6) | 0.382c |
| Hemoglobin (g/dL) | 13.8 (1.8) | 14 (1.8) | 13.3 (1.8) | 0.163c |
| Platelets (×103/μL) | 243 (100) | 237 (88) | 269 (138) | 0.226c |
| D-dimer (×103ng/mL) | 2.4 (4.0) | 2.1 (3.8) | 3.8 (4.8) | 0.130c |
| GFR (mL/min) | 65.7 (22.5) | 68.7 (20.5) | 49.9 (25.3) | 0.001c |
| CRP (mg/L) | 134 (99) | 121 (91) | 193 (115) | 0.006c |
| LDH (IU/L) | 404 (268) | 355 (160) | 662 (504) | 0.041c |
| Plain thoracic x-ray | ||||
| Normal | 9 (9%) | 8 (10%) | 1 (6%) | 0.586a |
| Multilobular pattern | 49 (51%) | 43 (54%) | 6 (35%) | 0.122b |
| Extensive multilobular patterne | 18 (19%) | 11 (14%) | 7 (41%) | 0.016b |
| Others | 20 (21%) | 17 (22%) | 3 (18%) | 0.506b |
ARBs: angiotensin II receptor antagonists; EC: European Community; SD: standard deviation; DM: diabetes mellitus; CVD: cerebrovascular disease; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; GFR: glomerular filtration rate by CKD-EPI; ACEI: angiotensin-inhibiting enzyme inhibitors; LDH: lactate dehydrogenase; CRP: C-reactive protein; OSAHS: obstructive sleep apnea-hypopnea syndrome; SatO2: oxygen saturation.
The mean duration of symptoms upon admission was 8.85 days (range 1–27) and was less than two or three weeks in 50% and 91.3% of patients, respectively. The most frequent symptoms reported were fever (83%), dry cough (71%), asthenia (65%), and dyspnea (54%). Baseline SatO2 levels were less than 90% in 22% of patients. The chest x-ray was normal in 9.4% of patients. However, 19% presented with a multilobular pattern of involvement and 49% with an extensive multilobular pattern of involvement (defined as unilateral involvement of more than 50% or bilateral involvement of more than 25%). A chest CT scan was performed in seven patients and a lung ultrasound in 12 patients. Upon admission, the mean lymphocyte count was low and mean levels of D-dimer, PCR, and LDH were high. Ferritin levels were only available in 62 cases and interleukin-6 levels in 42 cases; they had a mean (SD) concentration of 1,037ng/mL (SD: 1,002) and 73.1pg/mL (SD: 191.5), respectively.
A COVID-19 diagnosis was established via SARS-CoV-2 PCR in 92 patients (95.8%). The four patients with negative SARS-CoV-2 PCR tests had serological tests that were positive for SARS-CoV-2, with simultaneous IgG and IgM, in all four cases. The SARS-CoV-2 PCR was positive in the nasopharyngeal sample in 81 of 94 cases (86.2%) and in sputum in 34 of 79 cases (43.03%). Four bronchoscopies were performed, with positive results on all SARS-CoV-2 PCR tests of the bronchoaspiration. Of the 13 patients with a negative SARS-CoV-2 PCR on the nasopharyngeal sample, eight had a positive SARS-CoV-2 PCR on the sputum sample and four had a positive serology (simultaneous IgG and IgM). The two patients from whom a nasopharyngeal sample was not taken had a positive SARS-CoV-2 PCR test on the sputum sample.
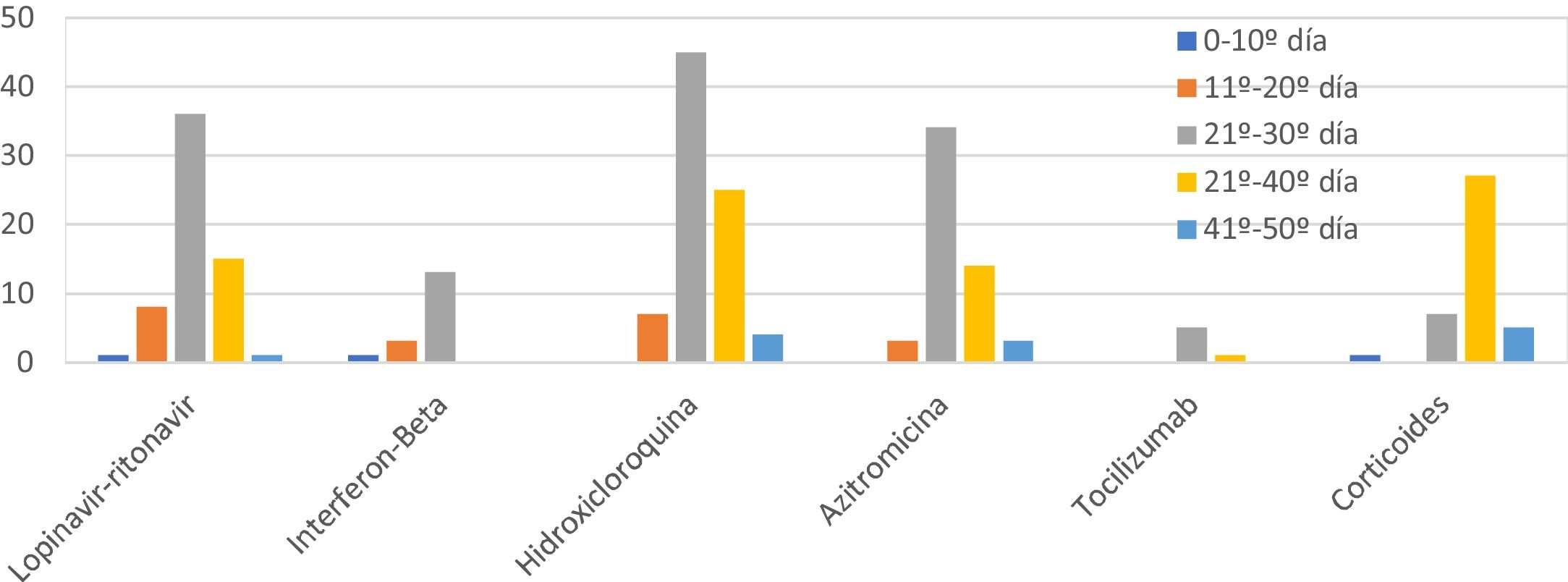
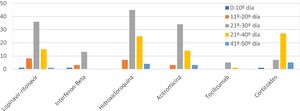
The combinations of drugs used varied during the study due to the emergence of new protocols (Fig. 2). The most used drug was hydroxychloroquine (85.4%), followed by lopinavir/ritonavir (61.5%), azithromycin (58.3%), interferon beta-1B (IFNb) (17.7%), and tocilizumab (6.3%). 39.6% of patients received corticosteroids at doses equivalent to ≥80mg/day of methylprednisolone. The most frequent combination was hydroxychloroquine with azithromycin and lopinavir-ritonavir (31, combined with IFNb in 11 of them), followed by hydroxychloroquine with azithromycin in 24 cases and lopinavir/ritonavir with hydroxychloroquine in 26. The mean duration of treatment was: hydroxychloroquine 8 days, lopinavir/ritonavir 10 days, azithromycin 5 days, IFNb 9 days, and corticosteroids 9 days. No significant arrhythmias occurred.
High-flow oxygen therapy was needed in 13 patients (13.5%), non-invasive mechanical ventilation in one patient (1.04%), and invasive mechanical ventilation in 18 (18.75%); the mean duration of the latter was 13 days.
Of the 96 patients included, 79 (82.3%) were discharged after recovery. The other 17 patients (17.7%) died after a mean hospital stay of 7.88 days (between 1 and 33 days). The most frequent cause of death was respiratory distress, which occurred in 14 patients (82.3%), followed by multiple organ dysfunction syndrome in two (11.8%) and sepsis in one (5.9%). No acute ischemic cardiac events or arrhythmias were identified. The mean overall hospital stay was 11 days and among survivors, 12.03 days. Eighteen patients required admission to the ICU (18.7%) and four died (22.2%). Up to 15 patients with COVID-19 were hospitalized in the ICU simultaneously. Thirteen patients (13.5%) were hospitalized in the intermediate care unit (created ad hoc as an area for administering high-flow oxygen or non-invasive mechanical ventilation): three were transferred to the ICU and of the other ten, four died. Two patients died in the emergency department. Combined ICU-intermediate care unit mortality was 25.8%.
On the bivariate analysis, no statistically significant association was detected between mortality and gender. However, age, polypharmacy, and prior treatment with ACEIs or ARBs were significantly more frequent among those who died. An absence of comorbidities was present in just 6% of patients who died versus 43% of survivors (p=0.004). Among those who died, there were significantly higher rates of hypertension, cerebrovascular disease, and cardiopathy; worse mean baseline SatO2 levels; lower glomerular filtration rates; and higher mean concentrations of CRP and LDH. A significant relationship was not detected between mean lymphocyte count nor mean concentration of D-dimer and death. An extensive multilobular pattern of involvement was significantly more frequent among those who died (Table 1).
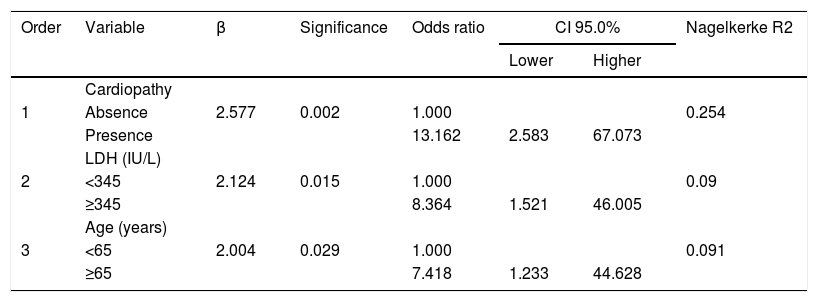
On the multivariate model, variables found to be statistically significant were included in the analysis of the raw data. Continuous variables were dichotomized at the median value. Adjustments were made with the independent risk factors for mortality of presence of cardiopathy, with an OR of 13.162 (CI 95%: 2.583–67.073); LDH levels ≥345 IU/L, with a OR of 8.364 (CI 95%: 1.521-46.005); and age ≥65 years, with an OR of 7.418 (CI 95%: 1.233–44.628). The model showed a correct adjustment (p=0.994) and an explained variance (Nagelkerke R2) of 43.5% (Table 2).
Final logistic regression model predictive of mortality during hospitalization, including the part of dependent variable variance explained for each variable (Nagelkerke R2).
| Order | Variable | β | Significance | Odds ratio | CI 95.0% | Nagelkerke R2 | |
|---|---|---|---|---|---|---|---|
| Lower | Higher | ||||||
| 1 | Cardiopathy | 0.254 | |||||
| Absence | 2.577 | 0.002 | 1.000 | ||||
| Presence | 13.162 | 2.583 | 67.073 | ||||
| 2 | LDH (IU/L) | 0.09 | |||||
| <345 | 2.124 | 0.015 | 1.000 | ||||
| ≥345 | 8.364 | 1.521 | 46.005 | ||||
| 3 | Age (years) | 0.091 | |||||
| <65 | 2.004 | 0.029 | 1.000 | ||||
| ≥65 | 7.418 | 1.233 | 44.628 | ||||
The start of the COVID-19 pandemic has led to the greatest acute overloading of the healthcare system in the history of Spain’s National Health System. It has forced hospitals to reorganize spaces and tasks and to cancel or delay the majority of scheduled activities. The high proportion of severe patients overwhelmed the capacity of the majority of hospitals. In this context, and without previous experience with this disease, it is essential to know what prognostic factors allow for the early identification of patients at risk of poor progress.
Our study shows that age, presence of cardiopathy, and admission LDH levels were associated with an elevated risk of death and explain the high proportion of deaths.
In the extensive literature that has already been generated on COVID-19, some prognostic factors for poor progress and mortality have been identified. However, a review of prognostic studies demonstrates that the majority of the works had methodological limitations:3 they excluded a significant proportion of patients who did not meet the outcome event (death or discharge), collected variables at different times,4 were based on information from a thoracic CT scan (not always available upon admission),5,6 or included variables that are difficult to obtain.7
From the first published series, it has been observed that advanced age and comorbidity entail a higher risk of death.8–10 A study from Wuhan showed that advanced age, high SOFA scale scores, and high D-dimer levels were risk factors for mortality.11 Another series from Wuhan found a relationship between mortality and comorbidity, secondary infection, and high levels of reactants.12 Other parameters that have been related with risk of death include elevated PCR9 or LDH levels,4,10,13 low lymphocyte4,10 or platelet counts,14 and low SatO2 upon admission.10
In our cohort, we confirmed that age is a very powerful risk factor. However, upon hospital admission, D-dimer levels were higher and SatO2 levels lower among patients who died, though the difference was not statistically significant. The LDH value was indeed identified as a significant variable. The first studies published reported that the comorbidities most frequently associated with the disease were hypertension, diabetes, and cardiopathy.11 Cardiovascular disease is particularly associated with an increase in mortality.15
As has been observed in all the published series, among our patients, the most frequent cause of death was acute respiratory distress syndrome. Myocardial damage, with secondary heart failure and arrhythmias, has been associated with an increase in mortality due to COVID-19,16 although in our series, there were no deaths due to cardiac causes.
Our study has several strengths. It is highly representative, as it includes 17 of the 21 total deaths (80.9%) that occurred in our hospital’s coverage area during the study period. Data were collected in a consecutive, prospective manner, excluding just five of the 101 patients due to the fact that they remained hospitalized. The mortality rate was low. This cannot be attributed to patients with mild cases being admitted, as of the 290 patients with positive SARS-CoV-2 PCR tests who consulted at the hospital, only 34.4% were admitted and of them, 29.2% required admission to the ICU or the intermediate care unit. This figure is similar to the 30% recently reported in a North American series17 and lower than the figures reported in the first series published in China.4
The mortality rate in the ICU was also similar to others that have been previously reported18,19 and much lower than the 61.5% reported in one of the first series published at the start of the pandemic.20 The overall mortality rate of our study is much lower than the first series published in Wuhan11 and similar to more recent series from China and the United States of America,15,16,21 although our patients had an older mean age. Finally, despite the low number of deaths we recorded, the model obtained explains the wide variance (43.5%) in in-hospital death.
One of the main limitations in establishing causal relationships among our variables and mortality, like in other series, is the heterogeneity of the treatments used, a consequence of evolving recommendations. On the other hand, it is likely that soon, better access to diagnostic testing and the emergence of effective, specific treatments may change the disease’s profile, favoring early diagnosis and better prognostic stratification.
ConclusionsOur model shows that a medical history of cardiopathy, LDH levels ≥345IU/L upon admission, and age ≥65 years are associated with greater in-hospital mortality due to COVID-19. This model must be validated in other cohorts.
Conflicts of interestThe authors declare that they do not have any conflicts of interest.
To the physicians María Luisa Hortas Nieto and Fernando Fernández Sánchez, who contributed to conducting the study; to Francisco Rivas Ruiz, for support with the statistical analysis; to Javier García Alegría and María Dolores Martín Escalante, for reviewing the manuscript.
Please cite this article as: Martos Pérez F, Luque del Pino J, Jiménez García N, Mora Ruiz E, Asencio Méndez C, García Jiménez JM, et al. Comorbilidad y factores pronósticos al ingreso en una cohorte COVID-19 de un hospital general. Rev Clin Esp. 2021;221:528–535.