Vascular disease (VD) is the most frequent cause of morbidity and mortality and its prevalence increases with age. Old patients are not included in studies on VD, their characteristics and treatments being unknown.
ObjectiveKnow the clinical characteristics of nonagenarian patients hospitalized in Internal Medicine services with a diagnosis of established VD and the adequacy of their pharmacological management.
Material and methodsThe NONAVASC-2 registry is an observational, prospective, multicentre study. Hospitalized patients for any cause were included. Data collection was carried out through an anonymous online database with sociodemographic, clinical, analytical, therapeutic and evolutionary parameters.
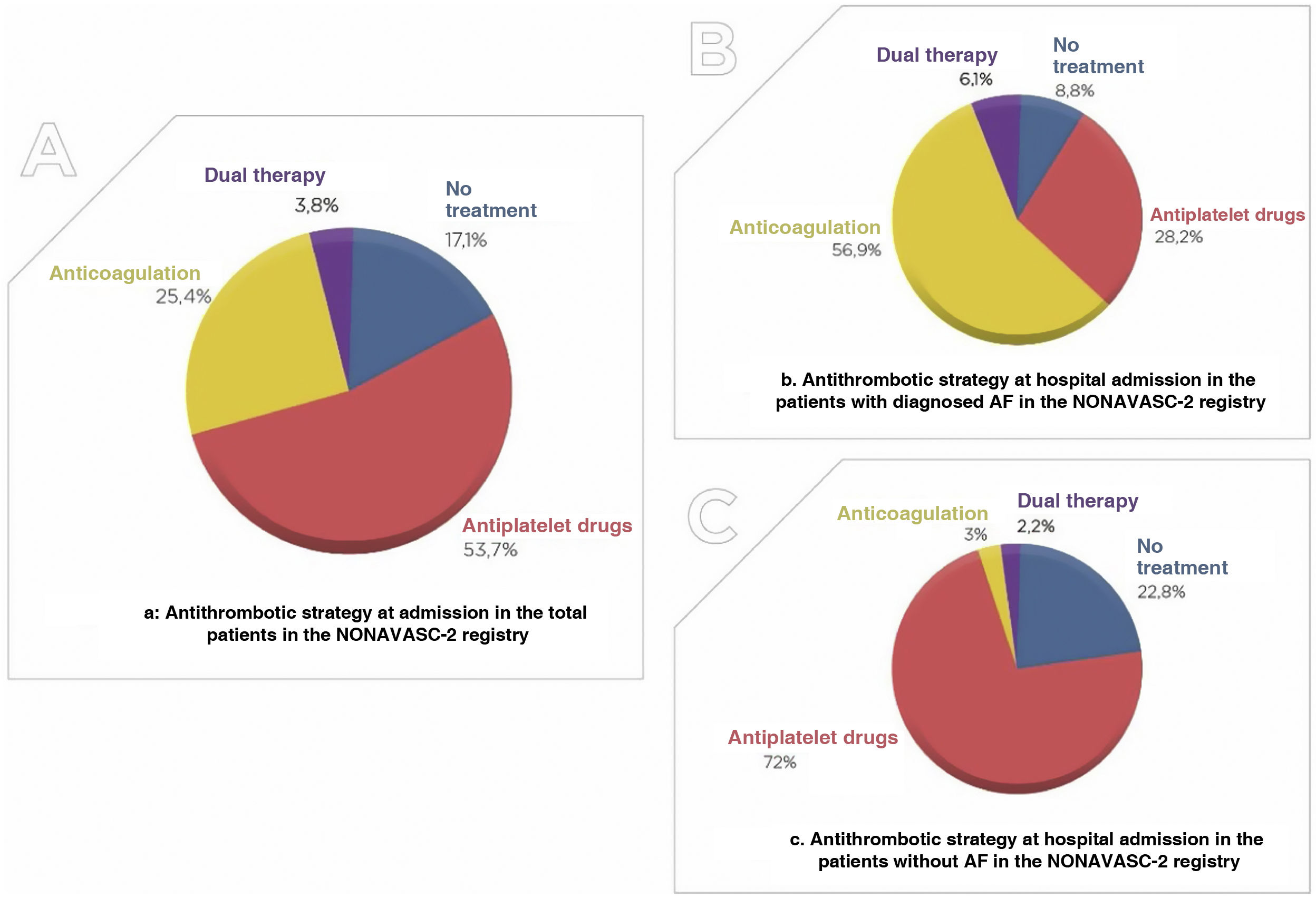
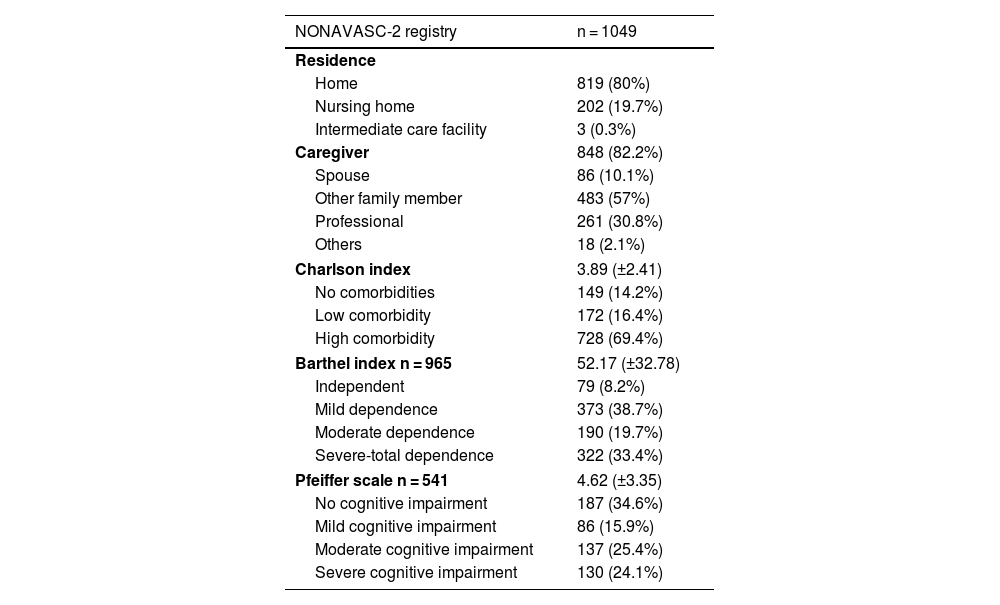
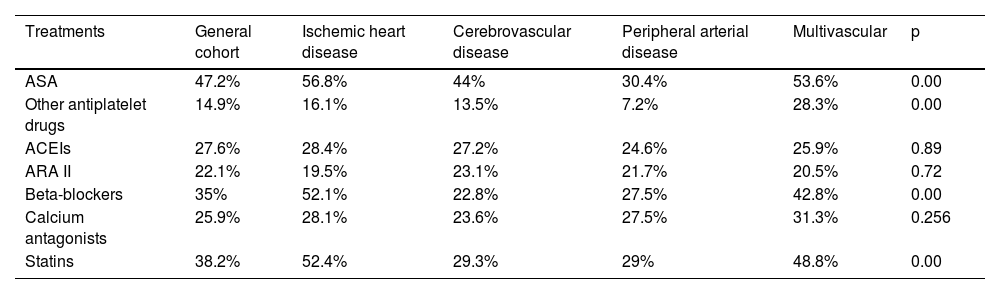
ResultsOne thousand forty-nine patients with a mean age of 93.14 years (57.8% women) were included. The prevalence of risk factors and VD was high: hypertension (84.9%), dyslipidemia (50.9%) and diabetes mellitus (29.4%). 33.4% presented severe-total dependency. 82.9% received antithrombotic treatment (53.7% antiplatelets, 25.4% anticoagulation and 3.8% double therapy). Only 38.2% received statins. The percentage of severe dependence (39.2% vs 24.1%; p = 0.00) and severe cognitive impairment (30.8% vs 13.8%; p = 0.00) was significantly higher among patients who did not receive them. 19% died during admission.
ConclusionsNonagenarian patients with VD present high comorbidity, dependence and mortality. Despite being in secondary prevention, 17% did not receive antithrombotics and only 38% received statins. The underprescription is conditioned, among other factors, by the functional status. More studies are necessary to determine the impact of this issue on their prognosis.
La enfermedad vascular (EV) es la causa más frecuente de morbimortalidad, y su prevalencia incrementa con la edad. Los pacientes muy añosos no se encuentran incluidos en los estudios sobre EV, desconociéndose sus características y tratamientos.
ObjetivoConocer las características clínicas de los pacientes nonagenarios hospitalizados en servicios de medicina interna con diagnóstico de EV establecida y la adecuación de su manejo farmacológico.
Material y métodosEl Registro NONAVASC-2 es un estudio observacional, prospectivo y multicéntrico. Se incluyeron pacientes hospitalizados por cualquier causa. La recogida de datos se realizó a través de una base anonimizada online con parámetros sociodemográficos, clínicos, analíticos, terapéuticos y evolutivos.
ResultadosSe incluyeron 1.049 pacientes con una edad media de 93,14 años (57,8% mujeres). La prevalencia de los factores de riesgo fue muy elevada: hipertensión (84,9%), dislipemia (50,9%) y diabetes mellitus (29,4%). El 33,4% presentaba dependencia grave/total. El 82,9% recibía tratamiento antitrombótico (53,7% antiagregantes, 25,4% anticoagulación y 3,8% doble terapia). Solo el 38,2% recibía estatinas. El porcentaje de dependencia (39,2 vs. 24,1%; p = 0,00) y deterioro cognitivo grave (30,8 vs. 13,8%; p = 0,00) era significativamente mayor entre los pacientes que no las recibían. El 19% falleció durante el ingreso.
ConclusiónLos pacientes nonagenarios con EV presentan una elevada comorbilidad, dependencia y mortalidad. A pesar de estar en prevención secundaria, el 17% de ellos no recibía antitrombóticos y solo el 38% estatinas. Esta infraprescripción está condicionada por la situación funcional, entre otros factores, por lo que es necesario realizar más estudios para conocer el impacto sobre su pronóstico.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.
Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<