New lipid-lowering strategies are needed for patients who do not achieve their recommended low-density lipoprotein cholesterol (LDL-C) goals with currently available lipid-lowering therapies. This may be even more relevant in patients with higher cardiovascular risk, including patients with type 2 diabetes (DM2).
Bempedoic acid is a non-statin lipid-lowering drug developed to treat hypercholesterolemia1. This new agent reduces cholesterol synthesis by inhibiting adenosine triphosphate citrate lyase, an enzyme upstream from 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Though several phase 2 and 3 clinical trials have demonstrated the lipid-lowering efficacy of bempedoic acid, information for the population with DM2 is more limited.
Some studies have shown that patients with DM2 present higher cholesterol absorption at the intestinal level and, conversely, lower cholesterol synthesis in the liver. This could be verified in other studies showing that patients with DM2 are hypo-responders to statins and hyper-responders to ezetimibe2,3. In addition, the published data strongly suggest that patients with DM2 may be more likely to benefit from ezetimibe/statin combination therapy.
This study aims to verify using a meta-analysis technique if the lipid-lowering response to bempedoic acid was similar in patients with or without DM2.
A systematic review was conducted based on the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist4. Two independent reviewers searched the electronic PubMed/MEDLINE, Embase, Science Direct, Scopus, and Cochrane Controlled Trials databases for the terms “bempedoic acid,” “ETC-1002,” and “diabetes.”
Our meta-analysis included randomized trials of bempedoic acid therapy (180 mg/day) versus placebo which reported LDL-C with a minimum of 4 weeks of follow-up in a subgroup of patients with DM2. Potential risks of bias were evaluated for all included studies using the revised Cochrane Risk-of-Bias tool for randomized trials (RoB 2)5.
The primary endpoint was defined as the percentage change in LDL-C levels, comparing subject groups with bempedoic acid versus placebo. Measures of effect size were expressed as mean difference and all study-specific estimates were combined using the inverse variance method for pooling. The I2 statistic was calculated to quantify between trial heterogeneity and inconsistency. The random-effects model was chosen because heterogeneity was high (I2 > 40%).
The search included 136 potentially relevant articles after title screening, and 115 studies were excluded after abstract screening. After a full textual analysis, 16 studies were removed because they did not include the population, treatment, or outcomes relevant to our study. Five eligible trials of bempedoic acid (2404 patients) were included6–10. All the evaluated studies were randomized clinical trials and had a placebo group.
Regarding the quality of the studies, three of them showed a low risk of bias while the other two presented some concerns. Two studies included statin intolerant patients and three included subjects with atherosclerotic cardiovascular disease, heterozygous familial hypercholesterolemia, or both. In all the trials, the patients were eligible to participate if they had been taking stable doses of maximally tolerated statin therapy either alone or in combination with other lipid-lowering therapies and if they had an LDL-C level above the threshold defined in each study. In most cases, this LDL-C threshold ranged from 70 to 100 mg/dL in patients with cardiovascular disease or heterozygous familial hypercholesterolemia and from 100 to 130 mg/dL in subjects in primary prevention. The follow-up ranged between 12 and 24 weeks.
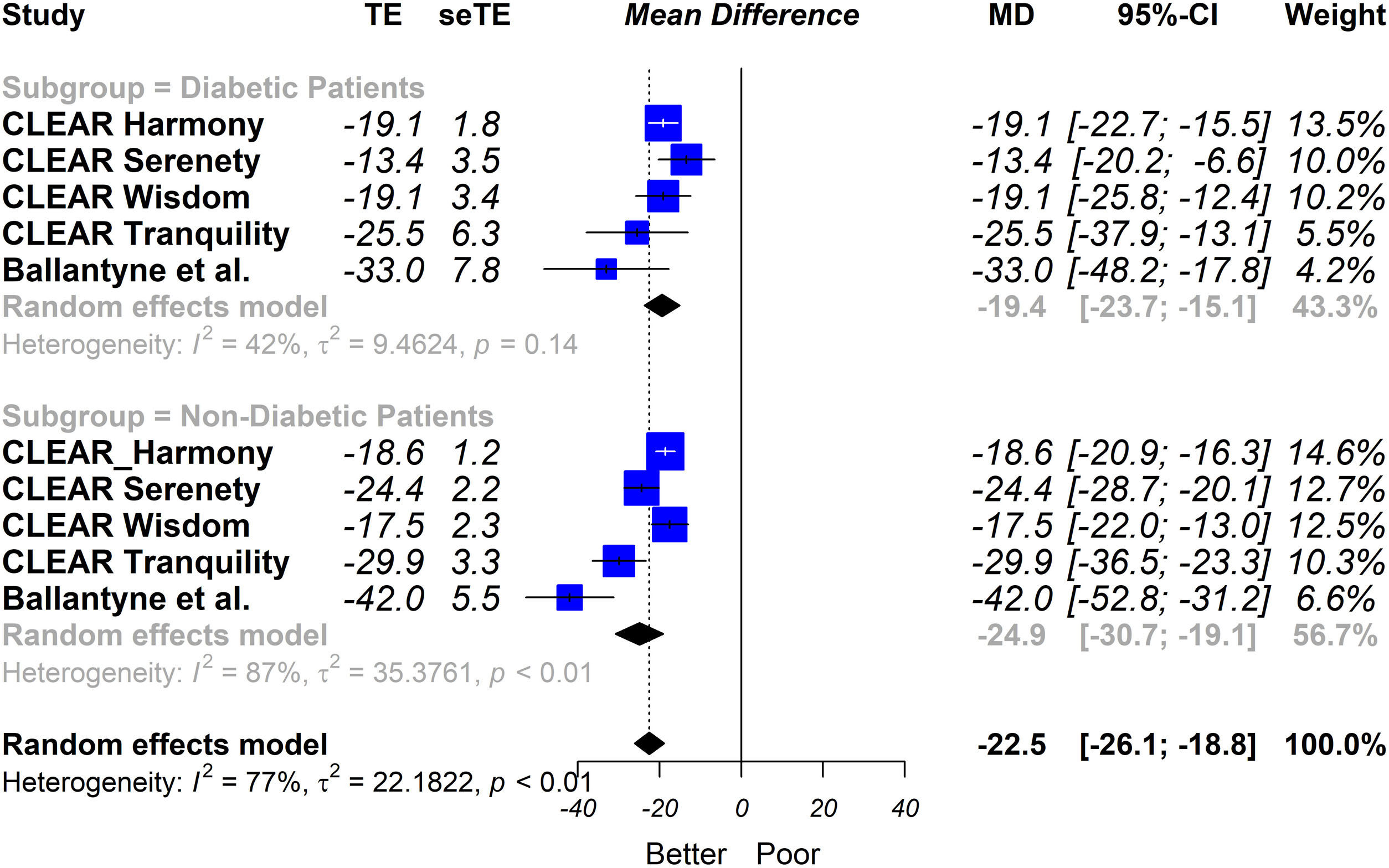
Our results showed that bempedoic acid was associated with a significant percentage of reduction in LDL-C levels (−19.4% [95% CI − 23.7% to −15.1%)]); I2 = 42% in patients with DM2. The results were similar to those observed in the population without DM2 (interaction p = 0.13) (Fig. 1).
Subgroup analysis (patients with or without type 2 diabetes) of the percentage change between bempedoic acid and placebo arms in the LDL-C level. Random effects model, mean difference, 95% confidence interval and I2 statistics.
MD: mean difference; seTE: standard error of treatment effect; TE: treatment effect.
Substantial residual cardiovascular risk and dyslipidemia remains present among patients receiving optimal ezetimibe/statin combination therapy, suggesting the possibility to improve therapy by adding other lipid-modifying therapies. The use of PCSK9 inhibitors is often limited by cost and access issues, therefore, the possibility of incorporating bempedoic acid into the management of diabetic dyslipidemia becomes relevant.
Response to a drug is not just a genetic or pharmacological phenomenon; it has a strong clinical impact. At least with statins, several studies show that hypo-responder patients experience more events and a worse prognosis. Due to the specific characteristics of cholesterol homeostasis observed in patients with DM2, understanding their response to new lipid-lowering drugs is necessary. In conclusion, the findings of our study suggest that the lipid-lowering response to bempedoic acid in patients with and without DM2 is similar.
Please cite this article as: Masson W, Barbagelata L, Lobo M, Nogueira JP. Ácido bempedoico en pacientes con diabetes tipo 2. Rev Clín Esp. 2022;222:251–253.