SARS-CoV-2 infection is associated with a high risk of malnutrition, mainly due to increased nutritional requirements and the presence of a severe and universal inflammatory state. Associated symptoms contribute to hyporexia, which perpetuates the negative nutritional balance. Furthermore, dysphagia, especially post-intubation, worsens, and makes intake unsafe. This risk is greater in elderly and multimorbid patients. Inflammation to varying degrees is the common link between COVID-19 and the onset of malnutrition, and it is more correct to refer to disease-related malnutrition (DRM). DRM worsens the poor prognosis of SARS-CoV-2 infection, especially in the most severe cases. Therefore, it is necessary to identify and treat people at risk early, avoiding overexposure and direct contact with the patient. We cannot forget the role that a healthy diet plays in both prevention and recovery after discharge.
La infección por SARS-CoV-2 se relaciona con un riesgo alto de malnutrición, principalmente por el aumento de los requerimientos nutricionales y la presencia de un estado inflamatorio severo y universal. Los síntomas asociados contribuyen a la hiporexia, que perpetua el balance nutricional negativo. Además, la disfagia, especialmente posintubación, empeora y hace poco segura la ingesta. Este riesgo es mayor en pacientes ancianos y multimórbidos. La inflamación en distinto grado es el nexo común entre la COVID-19 y la aparición de desnutrición, siendo más correcto hablar de desnutrición relacionada con la enfermedad (DRE). La DRE empeora el mal pronóstico de la infección por SARS-CoV-2, sobre todo, en los casos más severos. Por ello es necesario identificar y tratar precozmente a las personas en riesgo evitando la sobreexposición y el contacto directo con el paciente. No podemos olvidarnos del papel que juega la dieta saludable tanto en la prevención como en la recuperación tras el alta.
The World Health Organization declared the epidemic caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and the disease it causes, COVID-19, a global public health emergency.1 Since the first cases were identified in December 2019 to now, the entity has gone from being considered an infectious disease that is primarily respiratory to being thought of as a systemic disease that can severely compromise a patient's life.2
Mild symptoms occur in 80% of cases, but 15% of patients experience severe disease and the remaining 5% develop a critical illness with acute respiratory distress syndrome (ARDS) and multiple organ dysfunction syndrome. Up to 20% of patients require a prolonged stay in the intensive care unit (ICU), with the functional sequelae this entails.3
SARS-CoV-2 infection is related to a high risk of developing malnutrition, mainly due to an increase in nutritional requirements and the presence of a severe, generalized inflammatory state. Other symptoms such as cough, dyspnea, diarrhea, and loss of taste or smell contribute to a state of hyporexia that perpetuates the negative nutritional balance. Furthermore, dysphagia worsens—especially after extubation—and makes intake unsafe.4
Although COVID-19 can affect and cause severe disease in people of all age groups, special attention must be paid to patients who are elderly, who have multimorbidity, or who were previously malnourished, for whom risk of ICU admission is greater.5
Mortality due to COVID-19 in elderly patients is dramatic.6 Their vulnerability is linked to the biological “wear-and-tear” inherent to age, the greater prevalence of comorbidities, and the fact that up to 50% of elderly patients who need hospitalization present with varying degrees of malnutrition.7,8
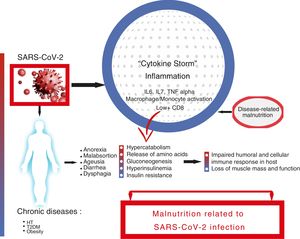
We have learned from other viral pandemics, such as MERS-CoV or Influenza A (H1N1), that risk factors for infectious diseases depend on the host, the pathogen, and the environment.9,10 Various factors associated with severity and a worse prognosis in COVID-19 have been described. They include age, presence of chronic diseases such as type 2 diabetes mellitus (T2DM), hypertension (HT), obesity, and diseases of the immune system.11,12 If there is anything that connects all of these, it is inflammation, to a greater or lesser degree.13 This exaggerated inflammatory response, called a “cytokine storm”, is the main trigger of a severe clinical presentation and death due to COVID-19.14,15
The “cytokine storm” is characterized on the one hand by elevated levels of proinflammatory cytokines, especially interleukins (IL) 6 and 7, and tumor necrosis factor (TNF) alpha, and on the other hand by macrophage activation syndrome; monocyte infiltration in the lung, liver, kidney, or lymph nodes; and severe lymphopenia.16–18 Elevated cytokine levels are negatively correlated with T lymphocyte levels, especially CD8+, and these in turn are correlated with the severity of the infection. It has been postulated that this severe depletion of cellular immunity could be related to lymphocyte displacement towards the inflamed tissues, but not with a direct infection of T cells by the virus.19
As stated above, the risk factors for infectious diseases also depend on the environment. Society has been moving away from a Mediterranean lifestyle towards more Western ways of living, in which diets rich in saturated fats, carbohydrates, and refined sugars and poor in fiber, antioxidants, and polyunsaturated facts are predominant. These diets are proinflammatory, induce lipotoxicity, and increase oxidative stress, activating the innate immune system through macrophages and neutrophils and inhibiting the adaptive immune system, altering the production and maturation of C.20,21
Lastly, without forgetting the other two actors—the pathogen and the environment—is the host. When speaking of malnutrition, we tend to refer to the condition related to starvation or inadequate intake, which is associated with an elevated morbidity and mortality. Malnutrition conditions disease and disease worsens malnutrition; thus it can correctly be referred to as a malnutrition-disease relationship.
However, in the context discussed herein, another concept must be added to the equation: inflammation. Therefore, it is more appropriate to speak of disease-related malnutrition (DRM). DRM is defined as an alteration of body composition caused by a nutritional deficit (due to decrease in intake or an increase in losses or requirements) as a consequence of an acute or chronic disease which suppresses vital functions and negatively affects clinical progress. DRM increases morbidity and mortality and worsens the prognosis, costs, and quality of life of patients.
In 2010, the European and American nutrition societies, ESPEN and ASPEN, published a consensus document defining DRM and starvation, highlighting the importance of the role of systemic inflammatory response, present to varying degrees in both acute and chronic illnesses, in the development of DRM.22
In 2019, The Global Leadership Initiative on Malnutrition (GLIM) released a consensus document for the diagnosis of DRM. It was based on phenotypic criteria (involuntary weight loss, low body mass index, or loss of muscle mass) to which, as a novelty, an etiological criterion was added, among which disease-associated inflammation was again included.23
The role of inflammation in both the pathophysiology of DRM and in a severe clinical presentation in COVID-19 appears obvious. The aggression, in our case SARS-CoV-2 infection, gives rise to an inflammatory response. This response is initially beneficial if well controlled, but in severe cases leads to the aforementioned “cytokine storm”, with severe metabolic consequences. With the increase in energy output, muscle amino acids are released that are used for gluconeogenesis and protein synthesis, which are highly necessary for the immune response and tissue repair.
In parallel, hormonal changes contribute to modifying the metabolic response. Hyperinsulinemia increases the production of ketone bodies and their use as a substrate in the brain. Eventually, this situation of hyperinsulinemia and insulin resistance worsens hyperglycemia, a factor known to increase morbidity and mortality,24 along with secondary hypertriglyceridemia, lipolysis, and fatty acid oxidation.25 The heart can lose up to 30% of its usual weight due to a decrease in myofibroblasts, necrosis, or inflammatory infiltrates. In the lungs, the number of alveolar macrophages and levels of pulmonary surfactant decrease, making the individual more prone to attack. Modifications occur in the synthesis and secretion of growth hormone, insulin, glucagon, and gonadotropins.26
The detrimental consequences the onset of DRM has on the clinical course of COVID-19 seem obvious. On the one hand, they are due to the onset of a defective, insufficient immune response. On the other, they are due to the onset of sarcopenia secondary to physical deconditioning that produces loss of muscle mass and functional capacity, perpetuating the cycle and slowing the patient’s recovery.27 (Fig. 1).
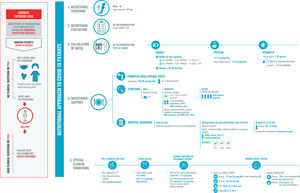
Identifying malnutrition risk and beginning appropriate, early nutritional support from the moment of first contact with a patient are fundamental pillars for improving the prognosis of COVID-19 patients.28,29 But how can we do this? We must adapt the universal algorithm for nutritional management to these clinical and epidemiological conditions.
The need for rigorous personal protection due to the high risk of transmission limits contact with the patient; the use of instrumentation for the evaluation of the patient’s nutritional state, dysphagia, or sarcopenia; and treatment. It is recommended to limit the time and frequency of contact and to avoid using reusable materials and procedures which could generate aerosols. Nevertheless, there is one tool within our reach that is easy to use and low-cost: the medical record, which our treatment plan should be based on and which allows us to determine a patient's needs, even with limited resources.
Adequate nutritional support must form part of the comprehensive treatment plan during the entire disease process. This nutritional plan must be re-evaluated and changed according to the calculation of a patient’s needs (Fig. 2), the presence of dysphagia (Fig. 3), and sarcopenia (Fig. 4). An appropriate nutritional state reduces complications, the length of the ICU and hospital stay, and sequelae.29
Recommendations for the nutritional approach to hospitalized COVID-19 patients.
Exposure must be safe, minimizing the risk of contagion. Therefore, we must be practical, take a proper medical history, and use disposable materials. The five fundamental points are:
1. Nutritional screening, for which the MNA-SF test (due to its simplicity and because patient contact is not necessary. Another test that is validated but not recommended because it entails close contact with the patient for calculating the BMI is the NRS-2002) and a calf diameter (cutoff point of 31cm) will be used. If possible, the nutritional screening will be performed in the first 48hours following admission.
2. Nutritional evaluation: medical history and a determination of malnutrition with an MNA-SF test score of less than 12 points.
3. Calculation of needs according to the patient’s habitual weight and ideal weight in the case of obesity.
4. Nutritional support: oral intake will be prioritized, optimizing it when possible. If intake is not adequate, it will be supplemented with two oral nutrition supplements that are high in calorie and protein density, especially if there is sarcopenia or if the patient is elderly with multimorbidity.
If reaching daily needs is severely endangered, total enteral nutrition will be used, avoiding tubes so long as it is possible. Continuity of care will be taken closely into account after discharge from the hospital. Oral intake will be reinforced by maintaining supplementation until the patient reaches 70% of his or her needs.
5. Take into account the most common clinical conditions of hospitalized patients, which may affect their nutritional state and the approach to it.
Abbreviations: Ca2+: elemental calcium; PPE: personal protective equipment; CH: carbohydrates; HMB: β-hydroxy β-methylbutyrate; K: potassium; MNA-SF: Mini Nutritional Assessment-Short Form; Na: sodium; EN: enteral nutrition; P: phosphorus; PCR: Polymerase chain reaction; NGT: nasogastric tube; Sat: saturated; Vit D: vitamin D.
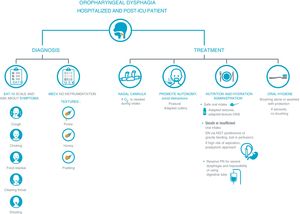
Recommendations for the approach to dysphagia in hospitalized and post-ICU COVID-19 patients.
In the approach to dysphagia, it is necessary to distinguish between diagnosis and treatment. The diagnosis will be made based on the clinical record and on the EAT-10 screening tool. The screening will be carried out at first contact with the patient, although given that direct contact is not recommended in these cases, using the MECV-V test in select patients can be considered. Treatment will be individualized and safe in order to avoid the generation of aerosols and to promote patient autonomy.
Proper oral hygiene must not be forgotten. We recommend prioritizing an oral diet adapted in composition and texture. In the event supplements are needed, they will be provided through specific supplements with adapted textures. If intake is not sufficient, the use of feeding tubes can be proposed, with a postpyloric approach in these cases. Parenteral nutrition will be reserved for severe cases or those in which it is impossible to use a gastric tube.
Abbreviations: EAT-10: Eating Assessment Tool; MECV: Volume-Viscosity Clinical Exploration Method; NP: parenteral nutrition; O2: oxygen; ONS: oral nutritional supplementation; NGT: nasogastric tube; ICU: intensive care unit.
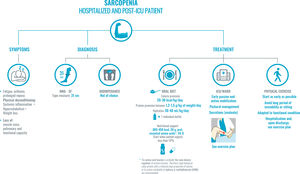
Recommendations for the approach to sarcopenia in hospitalized and post-ICU COVID-19 patients.
The diagnosis of sarcopenia will be based on the medical record. First, the common symptoms: fatigue and prolonged repose due to asthenia, hypoxia, or sedation which, along with systemic inflammatory symptoms, hypercatabolism, and significant weight loss, entail physical deconditioning.
In order to diagnose sarcopenia—and following the maxim of avoiding contact with the patient and/or use of non-disposable material as much as possible—the MNA-SF score and calf diameter (cutoff point of 31cm, which correlates well with lean mass and muscle strength) will be prioritized, as indicated in Fig. 1. Bioimpedance is not available in the majority of internal medicine departments. If it is available, it can be used, given that it is a simple technique that uses a portable, easy-to-clean device. Avoid techniques that entail a high risk of transmission, such as a DEXA scan, for example.
Treatment is based on two pillars: oral diet and early mobilization of the patient. First, the oral diet has to be adapted and have a high content of high biological value protein and energy density. If nutritional supplements are needed, those enriched with high biological value protein, HMB, and leucine will be prioritized, as they regulate protein turnover. Second, early mobilization of the patient: it will be active or passive, if incapacity is severe, and adapted to each patient’s functional condition.
Abbreviations: HMB: β-hydroxy β-methylbutyrate; MNA-SF: Mini Nutritional Assessment-Short Form; Prot: protein; ICU: intensive care unit; Vit. D: vitamin D.
The COVID-19 pandemic will leave us with a large number of recuperated patients with the same prior chronic conditions in addition to the sequelae of the infection. All will be susceptible to worsening if we do not focus on optimizing the patient's nutritional state during his or her hospitalization and upon discharge (Fig. 2).
Therefore, it is recommendable to take into account the role of chronic metabolic pathologies and the eating pattern in the susceptibility to and recovery from SARS-CoV-2 infection.30 Lastly, we cannot forget the third piece of the puzzle, physical exercise, always adapted to the patient’s functional condition.
FundingThe authors declare that they received no external funding for the creation of this manuscript.
Conflicts of interestThe content and recommendations of this document have been created based on the opinions of its authors with no interference from the sponsoring institution, the Spanish Society of Internal Medicine (SEMI). Said entity did not participate in any of the phases of design, decision-making, or creation of the material.
To Joana López Corduente and Sergio Carretero Gómez for their help in creating the infographics.
Please cite this article as: Carretero Gómez J, Mafé Nogueroles MC, Garrachón Vallo F, Escudero Álvarez E, Maciá Botejara E, Miramontes González JP. La inflamación, la desnutrición y la infección por SARS-CoV-2: una combinación nefasta. Rev Clin Esp. 2020;220:511–517.