Heart failure (HF) is a syndrome of epidemic proportions and one of the main reasons for hospital admission. Patient registries provide real-world clinical practice information which is complementary to clinical trials. RICA-2 is a registry of the Spanish Society of Internal Medicine. Its main goal is to know the clinical and epidemiological characteristics and prognostic factors of patients with HF treated in Internal Medicine Departments. The objective of this study is to present the design of the RICA-2, the baseline characteristics of the first 1000 patients included and their comparison with those of the historical cohort of the RICA registry.
MethodsObservational, multicentre and prospective study of patients with HF with the following inclusion criteria: age equal to or greater than 18 years old, diagnosis of HF according to the European Guidelines, indistinct inclusion in decompensation or stable phase, of patients with de novo HF or chronic HF, regardless of left ventricular ejection fraction, aetiology and comorbidities.
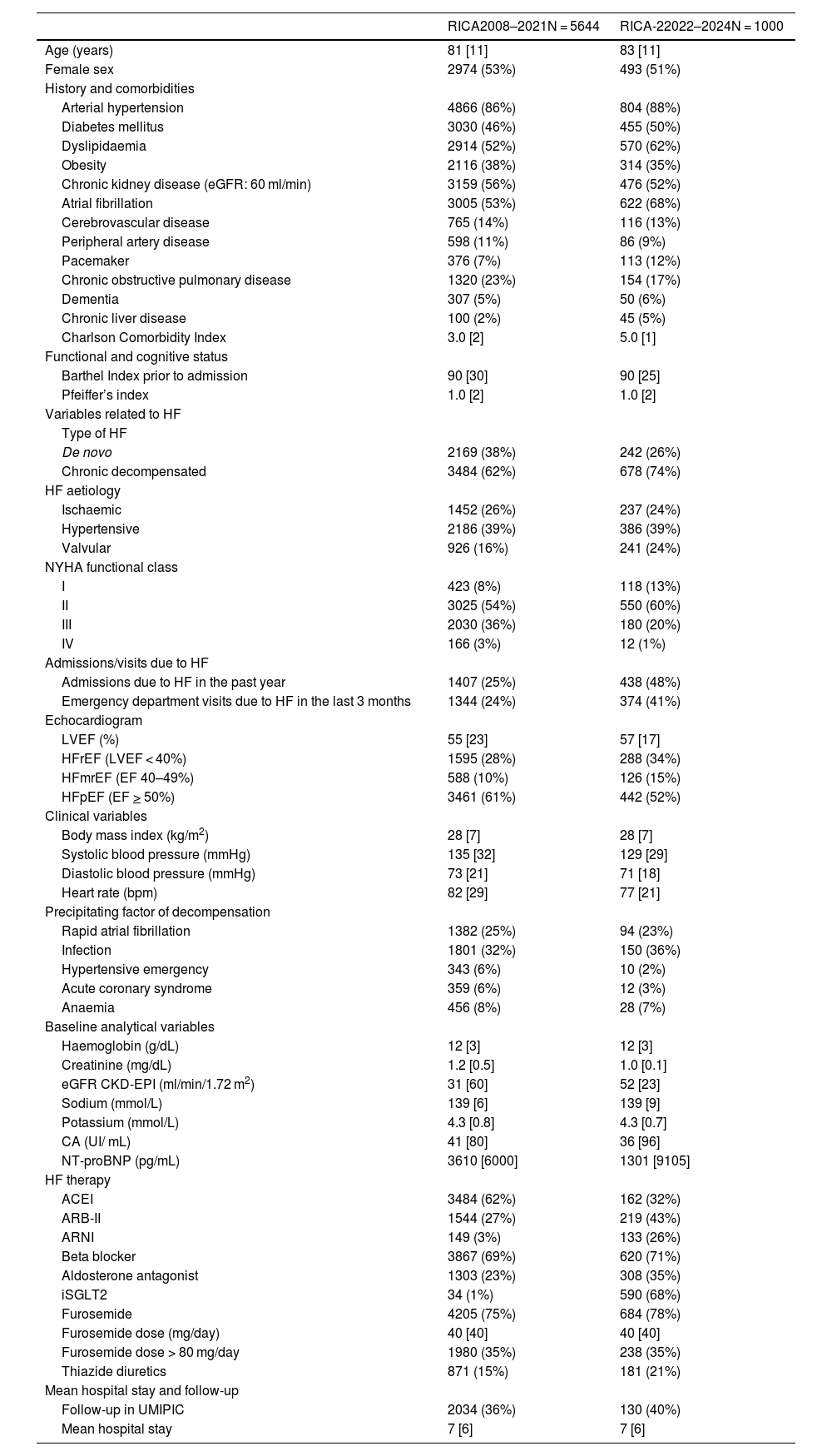
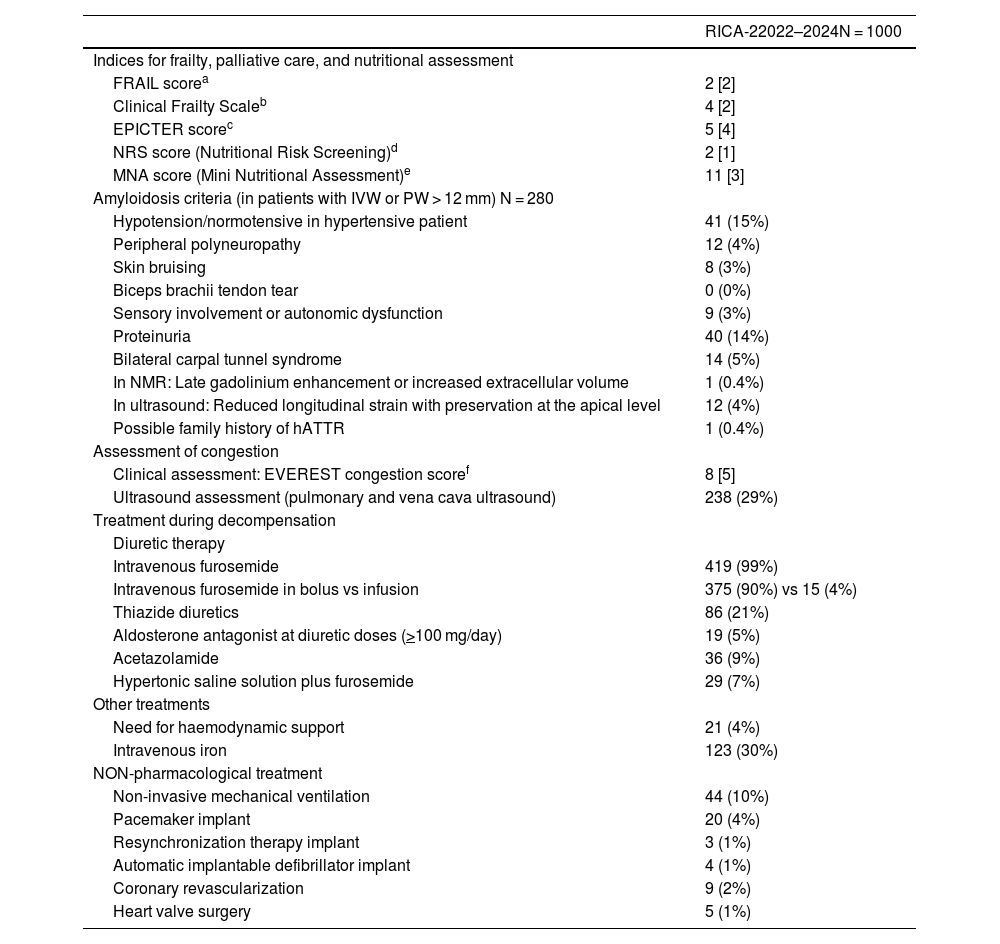
ResultsRICA-2 patients have advanced age (83 years old) and 51% are women. The comorbidity burden is higher than in the RICA registry (5 points in the Charlson comorbidity index), with predominating chronic decompensated HF (74%), hypertensive aetiology (39%) and preserved ejection fraction (52%). Most patients are pre-frail or vulnerable and are at risk of malnutrition.
ConclusionThe RICA-2 represents a contemporary cohort of patients that will provide us with clinical, epidemiological and prognostic information on patients with acute and chronic HF treated in Internal Medicine.
La insuficiencia cardíaca (IC) es un síndrome de proporciones epidémicas y uno de los principales motivos de ingreso hospitalario. Los registros de pacientes proporcionan información sobre la práctica clínica real complementaria a los ensayos clínicos. El RICA-2 es un registro de la Sociedad Española de Medicina Interna (MI) cuyo objetivo principal es conocer las características clínicas, epidemiológicas y factores pronósticos de los pacientes con IC atendidos en MI. El objetivo es presentar el diseño del RICA-2, las características basales de los primeros 1000 pacientes incluidos y su comparación con los de la cohorte histórica del registro RICA.
MétodosEstudio observacional, multicéntrico y prospectivo de pacientes con IC con los siguientes criterios de inclusión: edad igual o superior a 18 años, diagnóstico de IC según las Guías Europeas, inclusión indistinta en fase de descompensación o estable, de pacientes con IC «de debut» o con IC crónica, independientemente de la fracción de eyección del ventrículo izquierdo (FEVI), la etiología y las comorbilidades.
ResultadosLos pacientes del RICA-2 son de edad avanzada (83 años) y el 51% son mujeres. La comorbilidad es mayor que en el RICA (índice de Charlson de 5), predominando la IC crónica descompensada (74%), de etiología hipertensiva (39%) y FEVI preservada (52%). La mayoría de pacientes son pre-frágiles o vulnerables y están en riesgo de desnutrición.
ConclusiónEl registro RICA-2 representa una cohorte de pacientes contemporánea que nos aportará información clínica, epidemiológica y pronóstica de los pacientes con IC aguda y crónica atendidos en MI.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<