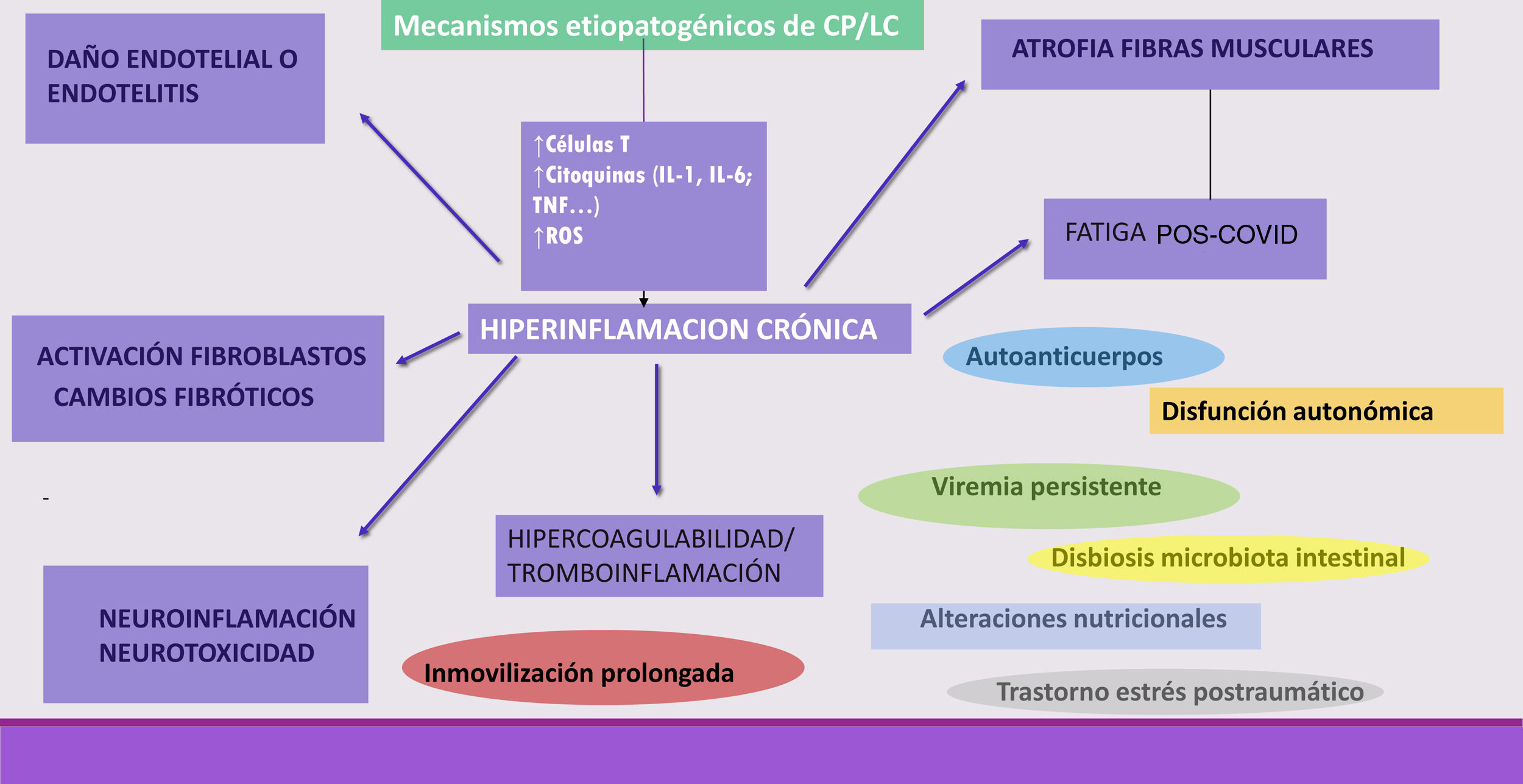
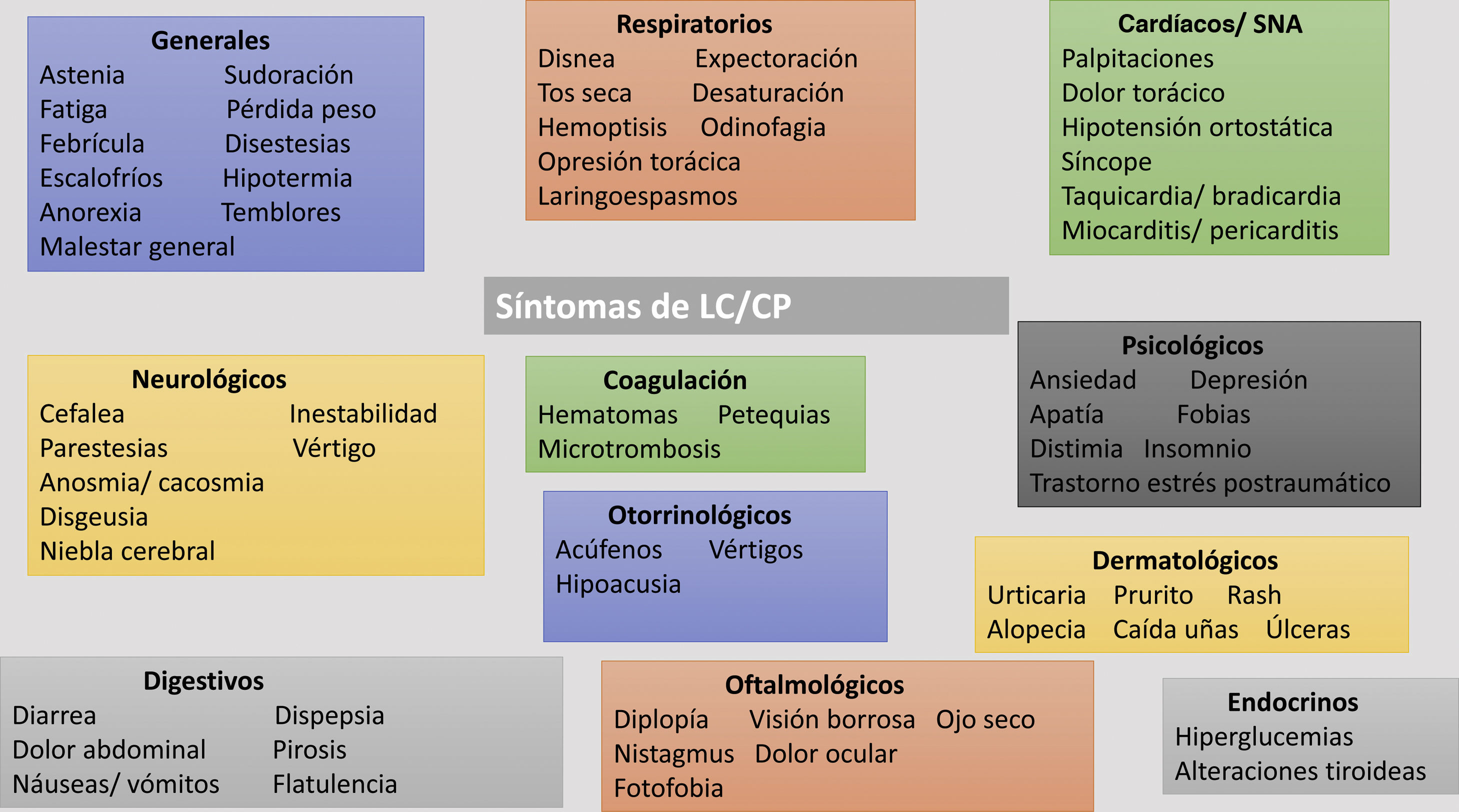
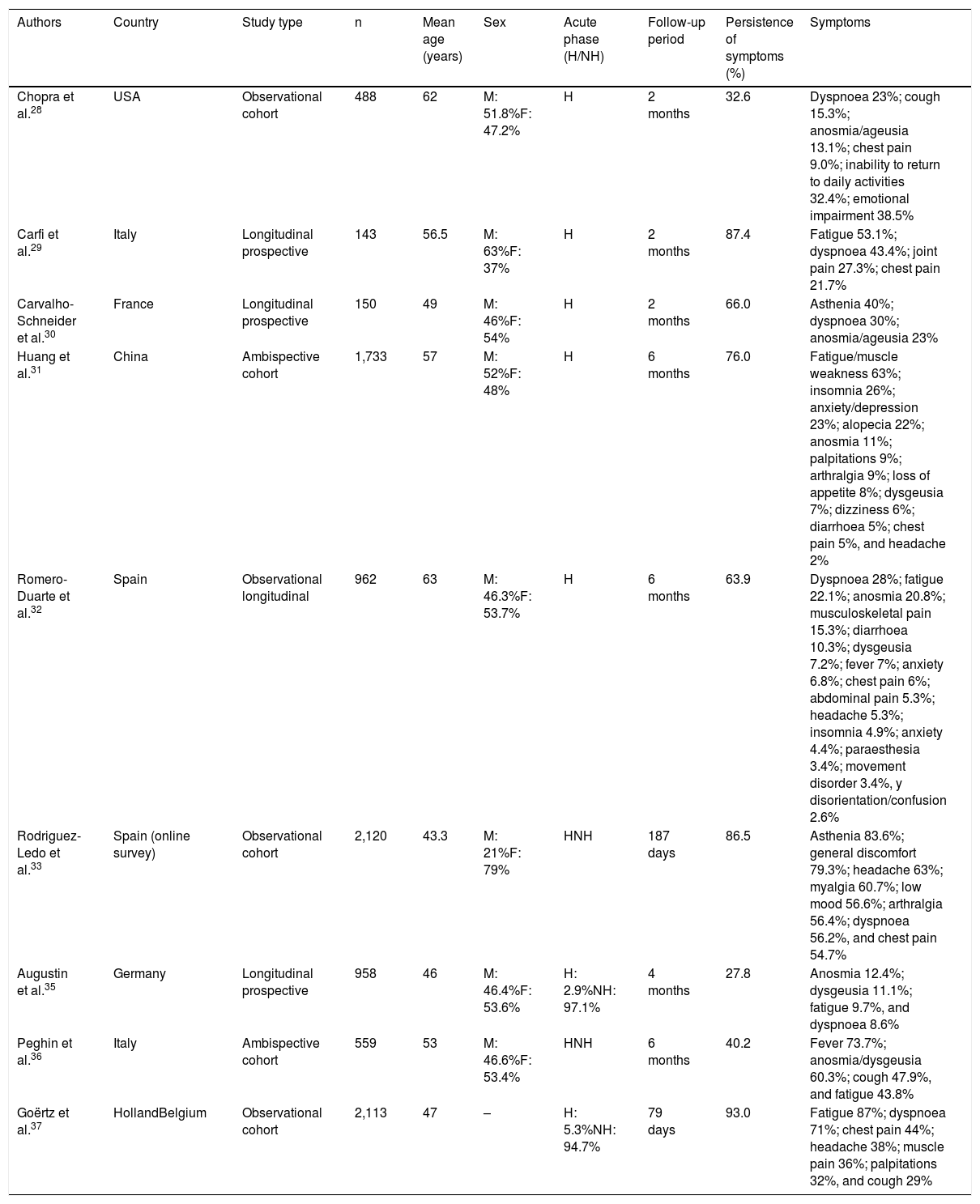
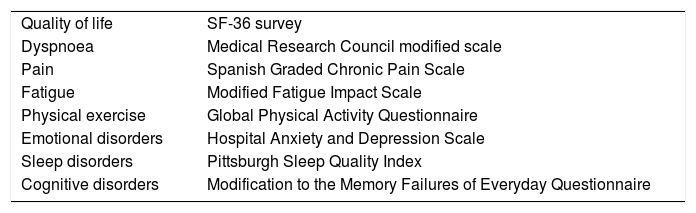
As the coronavirus-2019 disease (COVID-19) pandemic, caused by the infection with severe acute respiratory syndrome (SARS-CoV-2) coronavirus type 2, has progressed, persistent COVID-19 syndrome is an increasingly recognized problem on which a significant volume of medical literature is developing. Symptoms may be persistent or appear, after an asymptomatic period, weeks or months after the initial infection. The clinical picture is as markedly heterogeneous and multisystemic as in the acute phase, so multidisciplinary management is required. In addition, their appearance is not related to the severity of the initial infection, so they can affect both mild patients, even asymptomatic, and seriously ill patients who have required hospitalization. Although it can affect people of any age, it is more common in middle-aged women. The sequelae can generate a high impact on the quality of life, and in the work and social environment. The objective of this paper is to review persistent COVID-19 syndrome, to know its clinical manifestations and the strategies for the management and follow-up of these patients.
A medida que ha avanzado la pandemia de la enfermedad por coronavirus 2019 (COVID-19), originada por la infección por el coronavirus de tipo 2, causante del síndrome respiratorio agudo severo (SARS-CoV-2), el síndrome de COVID-19 persistente es un problema cada vez más reconocido y sobre el que se está desarrollando un importante volumen de publicaciones. Los síntomas pueden ser persistentes o aparecer, tras un periodo asintomático, semanas o meses después de la infección inicial. El cuadro clínico es tan marcadamente heterogéneo y multisistémico como en la fase aguda, por lo que se requiere un manejo multidisciplinar. Además, su aparición no está relacionada con la gravedad de la infección inicial, por lo que pueden afectar tanto a pacientes leves, incluso asintomáticos, como a enfermos graves que han requerido hospitalización. Aunque puede afectar a personas de cualquier edad, es más frecuente en mujeres de edad media. Las secuelas pueden generar un elevado impacto en la calidad de vida, y en el ámbito laboral y social. El objetivo de este trabajo es hacer una revisión sobre el síndrome de COVID-19 persistente, conocer sus manifestaciones clínicas y las estrategias para el manejo y seguimiento de estos pacientes.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<