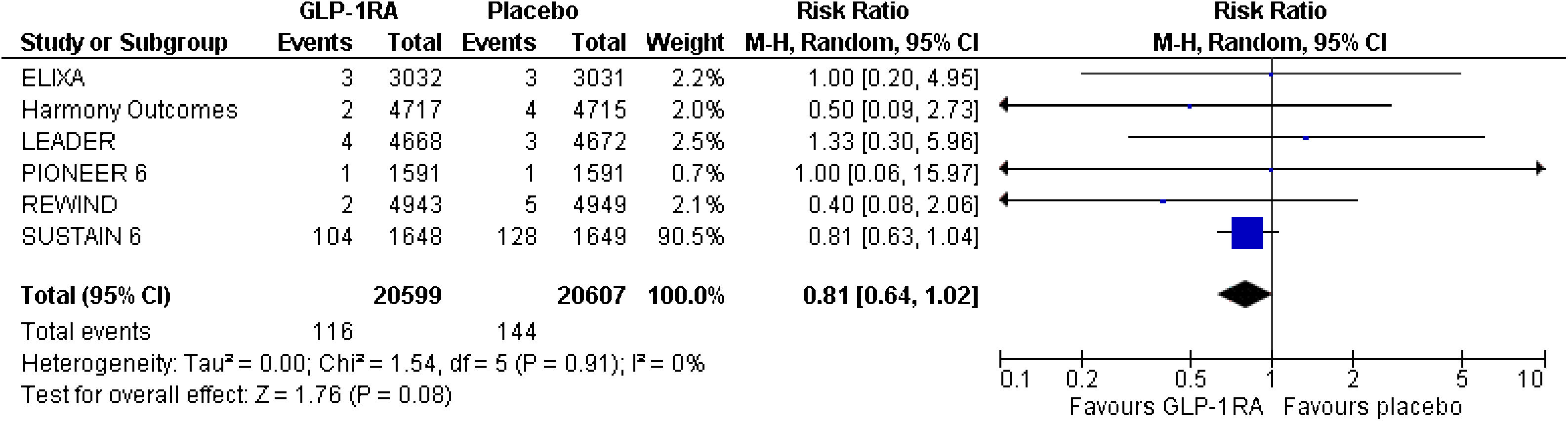
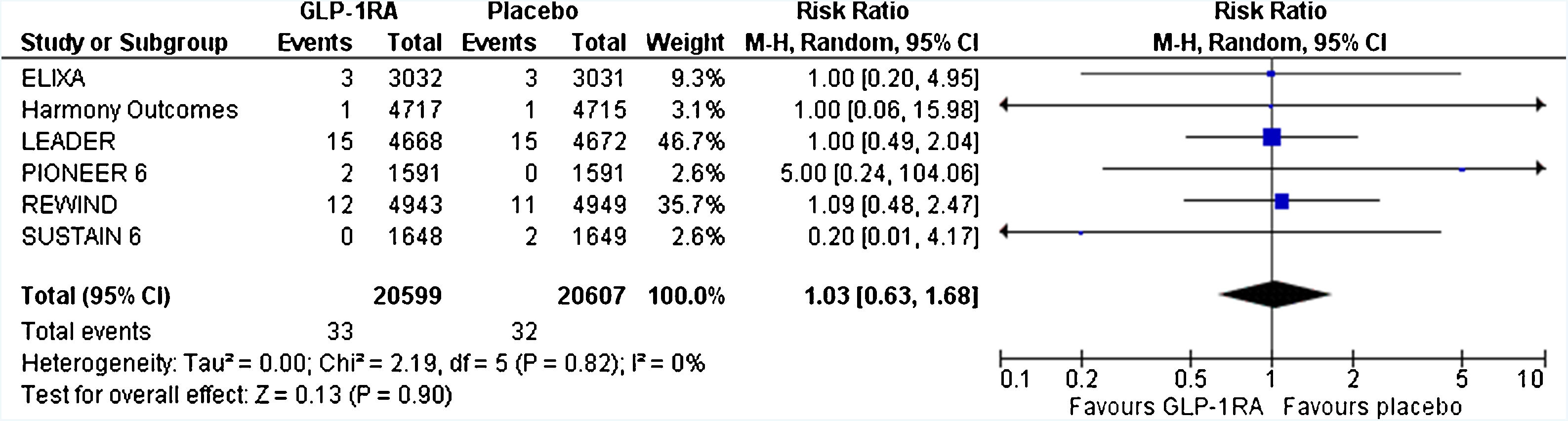
Patients with type 2 diabetes mellitus (T2DM) are at increased risk for severe coronavirus disease 2019 (COVID-19) and related mortality. Glucagon-like peptide-1 receptor agonists (GLP-1-RAs) have significant cardiovascular and renal benefits for patients with T2DM and related comorbidities. Their anti-inflammatory properties could be beneficial in these patients. This work provides less-biased estimates regarding the risk for respiratory tract infections and acute respiratory distress syndrome by performing the first significant meta-analysis of cardiovascular outcome trials in the literature. Notably, GLP-1-RAs do not seem to increase the risk for respiratory tract infection, pneumonia, or acute respiratory distress syndrome in patients with T2DM and cardiovascular comorbidities.
Los pacientes con diabetes mellitus tipo 2 (DMT2) presentan un mayor riesgo de sufrir una enfermedad grave por coronavirus 2019 (COVID-19) con un incremento de la mortalidad relacionada. Los agonistas del receptor del péptido similar al glucagón tipo 1 (AR-GLP-1) ejercen efectos cardiovasculares y renales beneficiosos en los pacientes con DMT2 de alto riesgo cardiovascular. Sus propiedades antiinflamatorias podrían resultar beneficiosas en estos pacientes. El presente estudio es un metaanálisis sobre el riesgo de infección respiratoria y distrés respiratorio del adulto causado por AR-GLP-1 utilizando como fuente los ensayos clínicos de seguridad cardiovascular publicados en la bibliografía. Hay que destacar que los AR-GLP-1 no parecen aumentar el riesgo de infección respiratoria, neumonía ni síndrome de distrés respiratorio del adulto en los pacientes con DMT2 y alto riesgo cadiovascular.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<