This work aims to describe the clinical characteristics and therapeutic management and to determine cardiovascular outcomes after one year of follow-up in a contemporaneous population with heart failure (HF) with and without type 2 diabetes in Spain. These factors were also analyzed in the DAPA-HF-like population (patients who met most inclusion criteria of the DAPA-HF trial) and in patients treated with SGLT2 inhibitors at baseline.
MethodsThis work is an observational, retrospective, population-based study using the BIG-PAC database. The index date was January 1, 2019. People aged ≥ 18 years who received care for HF in 2019 were selected. Events that occurred in 2019 were analyzed.
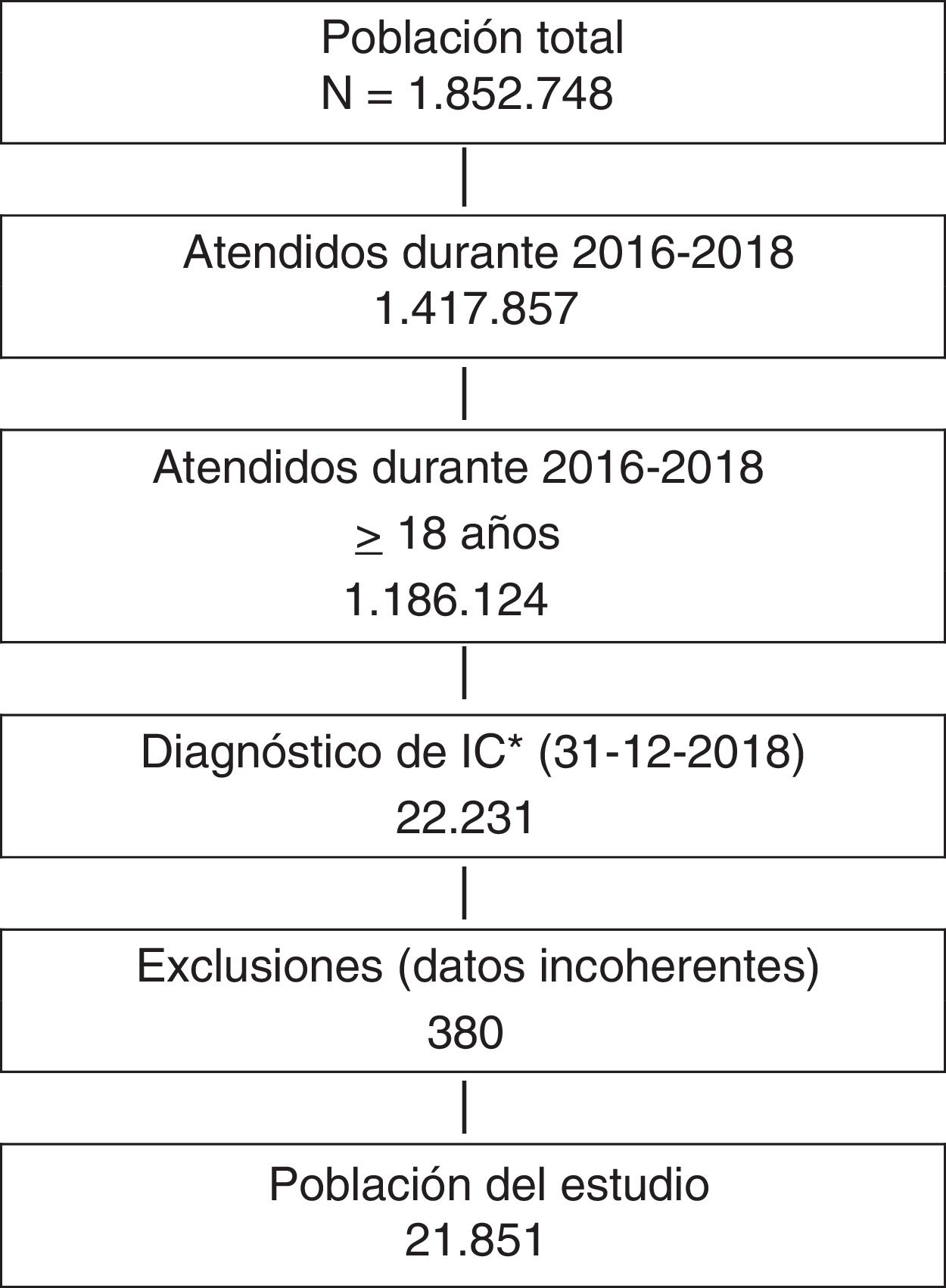
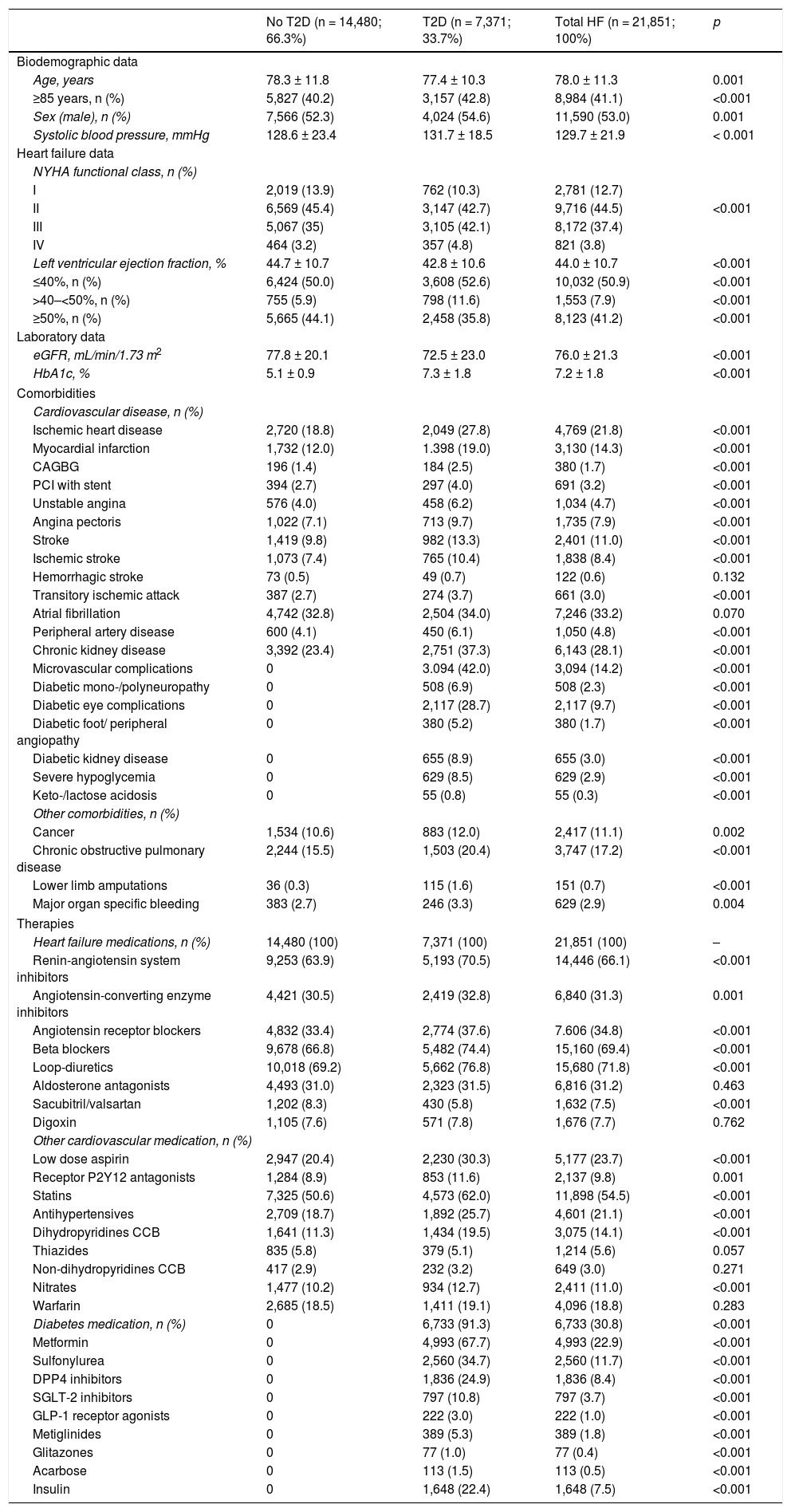
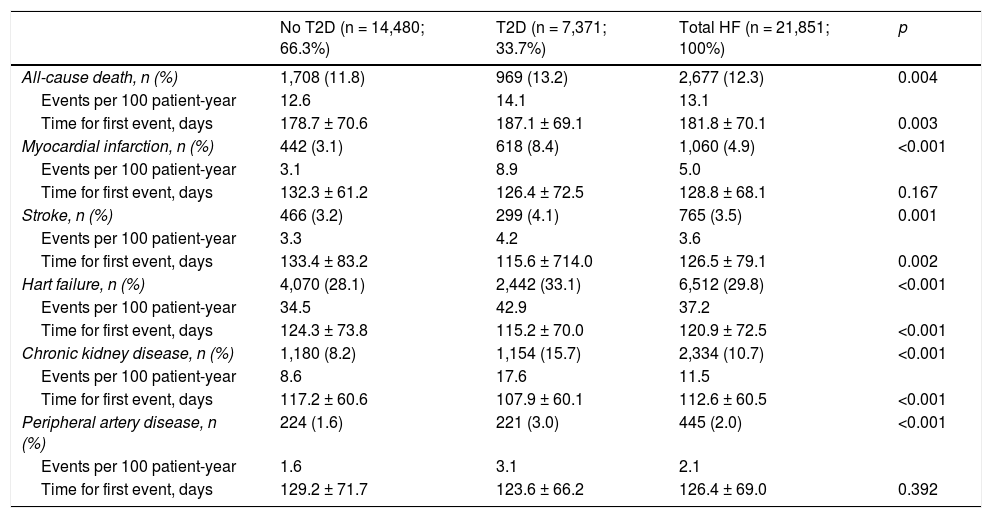
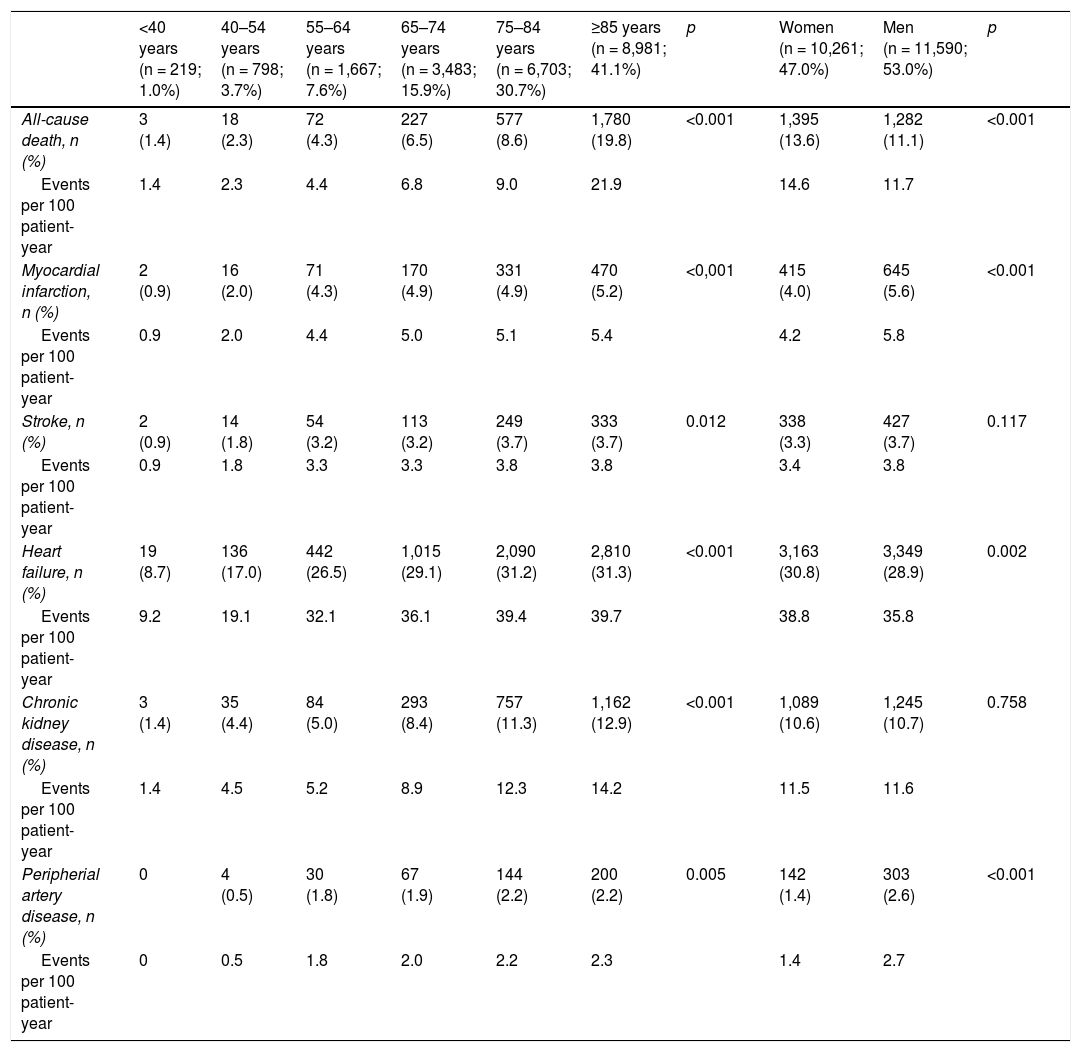
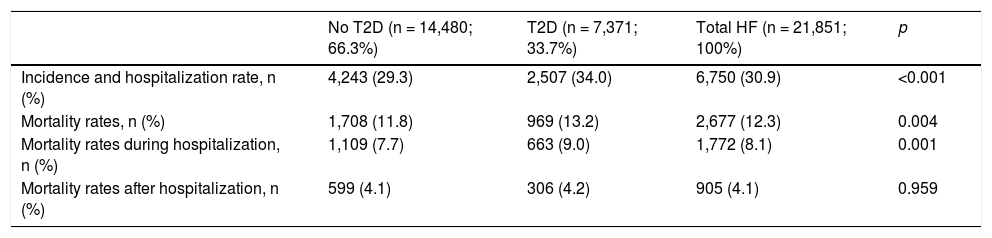
ResultsWe identified 21,851 patients with HF (age 78.0 ± 11.3 years, 53.0% men, 50.9% with HF with reduced left ventricular ejection fraction, 44.5% in NYHA functional class II). HF prevalence was 1.88% and incidence was 2.83 per 1,000 person-years. Regarding HF treatments, 66.1% were taking renin-angiotensin system inhibitors, 69.4% beta blockers, 31.2% aldosterone antagonists, and 7.5% sacubitril/valsartan. During the year of follow-up, 29.8% had HF decompensation which led to hospitalization (mean time to first event of 120.9 ± 72.5 days), 12.3% died, and 8.1% died during hospitalization. Events were more common among patients with type 2 diabetes. Hospitalizations for HF were more common in the DAPA-HF-like population.
ConclusionsIn Spain, the population with HF is elderly and has many comorbidities. Approximately half of patients have HF with reduced left ventricular ejection fraction. There is room for improvement in HF management, particularly through the use of drugs that reduce both HF hospitalization and mortality, in order to reduce the burden of HF.
Describir las características clínicas y el manejo terapéutico y determinar los eventos cardiovasculares tras un año de seguimiento en una población contemporánea con insuficiencia cardíaca (IC) con y sin diabetes tipo 2 en España. También se analizó en la población DAPA-HF (pacientes que cumplieron la mayoría de los criterios de inclusión del estudio DAPA-HF) y en los pacientes tratados basalmente con inhibidores SGLT2.
MétodosEstudio observacional, retrospectivo, poblacional, empleando la base de datos BIG-PAC. La fecha índice fue 1 de enero de 2019. Se seleccionaron sujetos ≥ 18 años que recibieron tratamiento por IC en 2019. Se analizaron los eventos durante 2019.
ResultadosSe identificaron 21.851 pacientes con IC (78 ± 11,3 años; 53% varones; 50,9% IC con fracción de eyección reducida; 44,5% en clase funcional NYHA II). La prevalencia de IC fue del 1,88% y la incidencia 2,83 por 1.000 pacientes-año. El 66,1% tomaba inhibidores del sistema renina-angiotensina, el 69,4% betabloqueantes, el 31,2% antialdosterónicos y el 7,5% sacubitrilo/valsartán. Durante el año de seguimiento, el 29,8% fue hospitalizado por descompensación de la IC (tiempo medio primer evento 120,9 ± 72,5 días), un 12,3% murieron, un 8,1% murieron durante la hospitalización. Los eventos fueron más frecuentes en los pacientes con diabetes tipo 2. Las hospitalizaciones por IC fueron más comunes en la población similar a DAPA-HF.
ConclusionesEn España, la población con IC es anciana y tiene muchas comorbilidades. Aproximadamente la mitad de los pacientes tienen IC con fracción de eyección reducida. Existe margen de mejora en el manejo de la IC, en particular mediante el empleo de aquellos fármacos que reducen tanto la hospitalización por IC como la mortalidad, para disminuir la carga de IC.
Article
Diríjase desde aquí a la web de la >>>FESEMI<<< e inicie sesión mediante el formulario que se encuentra en la barra superior, pulsando sobre el candado.

Una vez autentificado, en la misma web de FESEMI, en el menú superior, elija la opción deseada.

>>>FESEMI<<<