Since its emergence in December 2019, the coronavirus disease caused by the severe acute respiratory syndrome coronavirus 2 has become a global emergency, spreading rapidly worldwide. In response to the early referral of these patients to outpatient health centers, we decided to seek more effective treatments in the early stages of their referral. This study aims to prevent both the progression and deterioration of the physical conditions of COVID-19 patients, reduce the rate of referrals, and mitigate the risks of hospitalization and death.
Material and methodsConducted at Dibaj Therapeutic Center, Hamadan City, Iran, a double-blind randomized controlled trial encompassed 225 COVID-19 patients from April to September 2022. Ethical approval was obtained from Hamadan University of Medical Sciences (Approval No.: IR.UMSHA.REC.1400.957), with the protocol registered in the Iranian Registry of Clinical Trials (Registration No. : IRCT20220302054167N1). In this study, we included patients who tested positive for COVID-19- PCR and were symptomatic, excluding those who were pregnant or had received a COVID-19 vaccine. Patients with oxygen saturation above 92% were allocated to three groups: Group A received N-acetylcysteine, Group B received Bromhexine, and Group C received standard care. Follow-ups on oxygen levels, symptoms, and hospitalization needs were conducted on days 7 and 14, with hospitalized patients monitored for one month post-hospitalization.
ResultsThe study found that both N-acetylcysteine and Bromhexine can effectively reduce hospitalization rates and mortality and shorten the duration of hospitalization. The third visit of patients who received N-acetylcysteine showed an increase of 1.33% in oxygen saturation compared to their first visit, and in patients who received Bromhexine, this increase was 1.19%. The mortality rate was 9.33% in the control group and zero in both groups of patients who received medication.
ConclusionIn conclusion, the results of this study indicate that NAC and bromhexine may be effective in the treatment of patients with positive COVID-19, with a lower hospitalization rate, shorter hospitalization, faster recovery time, and reduced mortality compared to the control group.
Desde su aparición en diciembre de 2019, la enfermedad por coronavirus causada por el síndrome respiratorio agudo severo coronavirus 2 se ha convertido en una emergencia mundial, propagándose rápidamente por todo el mundo. En respuesta a la derivación temprana de estos pacientes a centros de salud ambulatorios, decidimos buscar tratamientos más eficaces en las primeras etapas de su derivación. Este estudio tiene como objetivo prevenir tanto la progresión como el deterioro de las condiciones físicas de los pacientes con COVID-19, reducir la tasa de derivaciones y mitigar los riesgos de hospitalización y muerte.
Material y métodosRealizado en el Centro Terapéutico Dibaj, ciudad de Hamadan, Irán, un ensayo controlado aleatorio doble ciego abarcó 225 pacientes con COVID-19 de abril a septiembre de 2022. Se obtuvo la aprobación ética de la Universidad de Ciencias Médicas de Hamadan (Aprobación No.: IR.UMSHA. REC.1400.957), con el protocolo registrado en el Registro Iraní de Ensayos Clínicos (Registration No. :: IRCT20220302054167N1). Los pacientes cumplieron con el diagnóstico de COVID-19 a través de la presentación de síntomas y la confirmación por PCR, excluyendo aquellos con antecedentes de vacunas y afectación de órganos. Los pacientes con una saturación de oxígeno superior al 92 % se asignaron a tres grupos: el grupo A recibió N-acetilcisteína, el grupo B recibió bromhexina y el grupo C recibió atención estándar. Los seguimientos de los niveles de oxígeno, los síntomas y las necesidades de hospitalización se realizaron los días 7 y 14, con pacientes hospitalizados monitoreados durante un mes después de la hospitalización.
Resultadosel estudio encontró que tanto la N-acetilcisteína como la bromhexina pueden reducir efectivamente las tasas de hospitalización y la mortalidad y acortar la duración de la hospitalización. La tercera visita de los pacientes que recibieron N-acetilcisteína mostró un aumento de 1,33% en la saturación de oxígeno en comparación con su primera visita, y en los pacientes que recibieron Bromhexina, este aumento fue de 1,19%. La tasa de mortalidad fue del 9,33% en el grupo control y nula en ambos grupos de pacientes que recibieron medicación.
ConclusiónEn conclusión, los resultados de este estudio indican que la NAC y la bromhexina pueden ser efectivas en el tratamiento de pacientes con covid-19 positivo, con una tasa de hospitalización más baja, una hospitalización más corta, un tiempo de recuperación más rápido y una mortalidad reducida en comparación con el grupo control.
Since its emergence in December 2019, the coronavirus disease (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global emergency, spread rapidly worldwide.1 While COVID-19 can present as mild symptoms, including fever, cough, and loss of smell or taste, it can also lead to severe cases with extensive lung involvement, acute respiratory distress syndrome, hospitalization, intubation, and even death.2 Such a situation declines the oxygen content in the blood of the patient. The production of cytokines and chemokines is one of the primary immune responses during viral infection.3 Large amounts of IL-8, a potent chemoattractant for neutrophils, have been reported in SARS patients.4,5 In severe COVID-19 patients, the rise in the number of neutrophils is associated with the disease severity.6 The production of high levels of proinflammatory cytokines leads to "cytokine storm".2,7 When the patient is admitted to the hospital, the disease is most likely to have advanced to the second or third stage, with respiratory problems and multiple organ failure. Therefore, from the second step onwards, we should look beyond the virus and focus on the cytokine storm and the free radical storm as pathogenic agents.8
N-acetylcysteine (NAC) has been long employed to treat paracetamol (acetaminophen) poisoning caused9 and as a mucolytic in chronic lung diseases. N-acetylcysteine is also an antioxidant and can reduce the oxidative stress7 as a prodrug, acetylcysteine is transformed into l-cysteine,10 which is the precursor of the biological antioxidant, glutathione. Therefore, the administration of N-acetylcysteine renews glutathione sources. N-acetylcysteine also has some anti-inflammatory effects through inhibiting NF-κB by activating nuclear factor kappa kinase regeneration and thus modulating cytokine synthesis. Replication of RNA viruses requires the support of an active NF-κB pathway in the host cells. Concerning human coronaviruses (HCoV-229E), suppression of NF-kB significantly reduces the replication rate. Thus, drugs capable of inhibiting NF-κB activation can decrement viral replication.2,11 Moreover, NAC has shown protective mechanisms against a variety of COVID-19-associated conditions, including cardiovascular diseases.12 Regarding cardiac injury and thrombosis as the potentially fatal complications of COVID-19, intravenous NAC has exhibited vasodilator, anti-inflammatory, and antiaggregatory effects of nitroglycerin which can be beneficial in the improvement of the outcomes, such as acute myocardial infarction, unstable angina, and acute pulmonary edema.13 In some studies, the NAC drug has also been recommended as a protective agent for reperfusion, helping to prevent endothelial tissue damage.14 Cardiovascular complications are common manifestations of both acute and post-acute sequelae (PASC) of COVID-19 infection. They encompass various cardiovascular disorders, including myocardial injury, acute coronary syndromes, myocarditis, cardiomyopathy, arrhythmias, heart failure (HF), and deep venous thrombosis.15
Bromhexine works by breaking down and thinning the mucous secretions in the respiratory tract, making it easier for the body to expel them. This helps to relieve symptoms such as cough, congestion, and difficulty breathing. Additionally, Bromhexine has been shown to have anti-inflammatory effects, which can help to reduce swelling and irritation in the respiratory tract.16 Bromhexine has a long history of use in respiratory tract disorders and has been widely studied for its effectiveness in treating these conditions. It is generally well-tolerated, with few side effects reported. Overall, Bromhexine is an important tool in the management of respiratory tract disorders characterized by thick and sticky mucous secretions. Its effectiveness as an expectorant and mucolytic agent makes it a valuable option for those seeking relief from respiratory symptoms.17
In the midst of the ongoing COVID-19 pandemic, preventing hospitalization and improving recovery among outpatients is of utmost importance. To address this issue, we have conducted a randomized clinical trial comparing the efficacy of N-Acetylcysteine with Bromhexine. Our goal was to find effective treatment options that could help manage the symptoms of COVID-19 as well as prevent disease progression and complications, potentially reducing the burden on hospital systems and mortality from COVID-19.
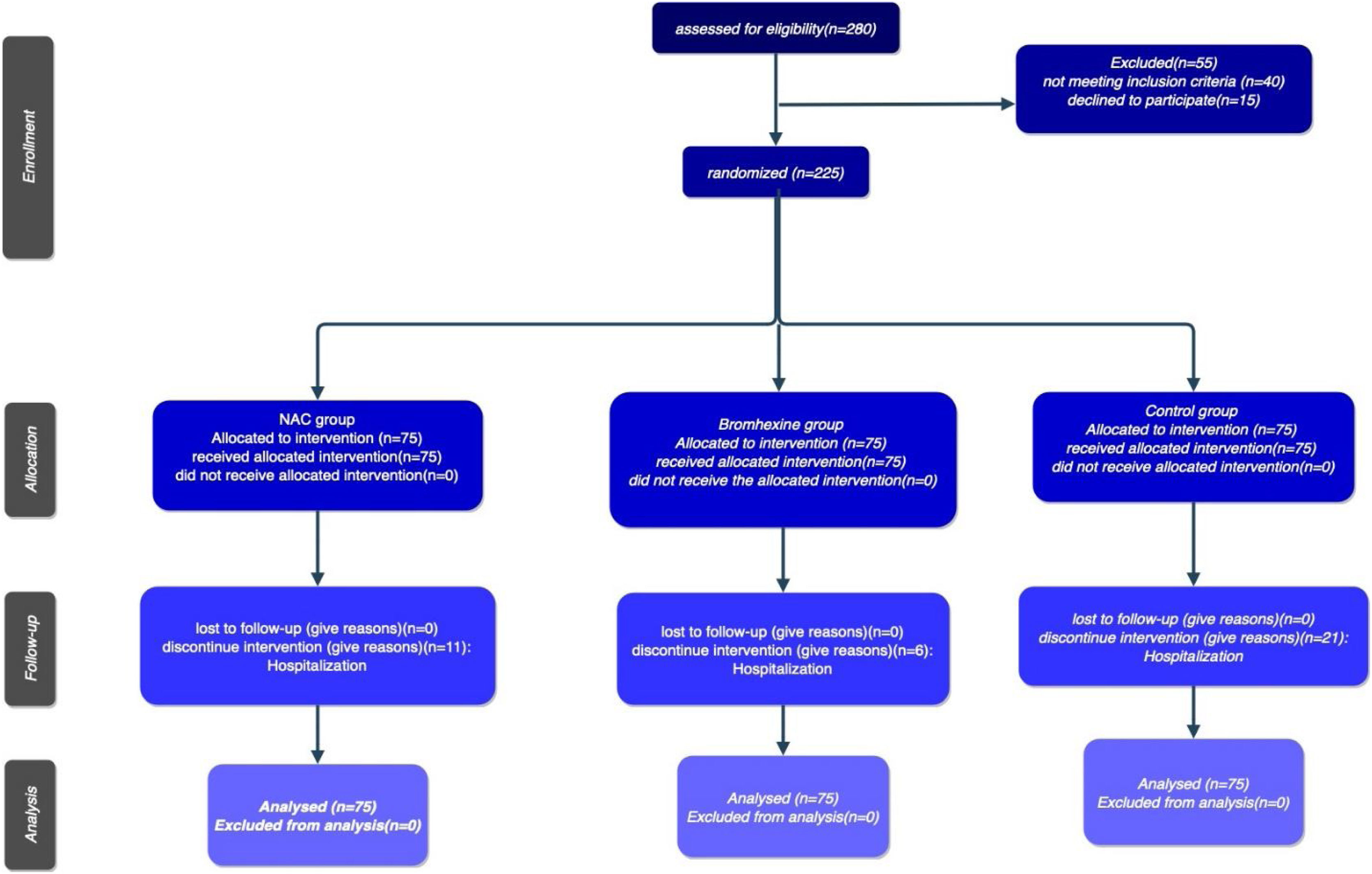
MethodsStudy design and settingA double blind randomized controlled trial was conducted on 225 outpatients who had undergone COVID-19 in Dibaj Therapeutic Center, Hamadan city, Iran. The entire study process was approved by the Research Ethics Committee of Hamadan University of Medical Sciences (No.: IR.UMSHA.REC.1400.957), and the protocol was registered by Iranian Registry of Clinical Trials (No.: IRCT20220302054167N1) between April 2022 and September 2022. The diagnosis of COVID-19 was based on the patients' presenting symptoms, which included cough, fever, weakness, lethargy, muscle pains, runny nose, and sore throat. Nasal and/or pharynx samples were collected from patients for an RT-PCR test to confirm the COVID-19 diagnosis at the health service center, and only PCR-positive patients were enrolled in the study. It is noteworthy that patients were included in the study only if there was no need for referral and no evidence of organ involvement including the lungs after Chest-CT on the initial visit, and they had never received the COVID-19 vaccine. At first, we measured the patient's o2 saturation with a pulse oximeter, and if o2 saturation was over 92%, 225 participants were then randomly assigned to three groups (A, B, and C). Group A received oral N-acetylcysteine 600 mg twice a day for five days, while group B received 8 mg Bromhexine tablets three times a day for five days. The control group (group C). The third group received only the routine and usual treatments prescribed for the other two groups. All patients took naproxen 250 mg twice daily for five days, famotidine 20 mg once daily for ten days, vitamin D 50,000 per week for four weeks, and vitamin C 1000 mg daily. Then, the patients were asked to return to the clinic on the 7th and 14th days to check their oxygen levels, monitor changes in symptoms, undergo necessary examinations, and be informed if hospitalization was required. All patients admitted to the hospital were followed up for one month after hospitalization, monitoring the duration of their stay, their general condition, and the mortality rate. The results of examinations and patients' characteristics were also recorded in a checklist designed for this purpose.
Objectives and hypothesesThe primary objective of the plan is to compare the effects of two drugs, acetylcysteine and bromhexine, on the rate of recovery and prevention of hospitalization in outpatients and mortality rate, with control group patients. The study aims to comprehensively investigate various facets concerning COVID-19 patients seeking care at the Dibaj Clinic. These include determining the frequency of COVID-19 among outpatients based on demographic information, assessing the variability in oxygen levels during clinic visits, and exploring the prevalence of symptoms at the time of visits. Additionally, the research involves comparing the average percentage of oxygen saturation before and after the use of N-acetylcysteine and Bromhexine, both on the 7th and 14th days of the disease. Furthermore, it investigates the frequency and duration of hospitalization among patients treated with N-acetylcysteine and Bromhexine, as well as control group patients. Hypotheses associated with these objectives address variations in incidence, oxygen levels, symptom prevalence, and hospitalization patterns across different groups and treatment modalities.
Sampling methodWe employed a six-block randomization method for patient allocation. Sheets of paper were prepared, with the letters "A" written on two sheets, "B" on two sheets, and "C" on two sheets. These were shuffled and placed in a desk drawer. At the time of the patient's eligibility, one sheet was randomly drawn, and the patient was assigned to group "A", "B", "C", or no drug. Notably, a specific sheet was not returned to the drawer until all six sheets had been drawn once. This random assignment process continued for the next six patients until the desired sample size of 225 patients was achieved.
Blinding methodAll three intervention and control groups received drugs with the same appearance, identified by the same code. Neither patients nor examiners were aware of the specific type of medication prescribed. As a result, the study was conducted in a double-blind manner."
Inclusion criteriaWe enrolled adult patients aged 18-80 years with symptoms suggestive of COVID-19 who presented to Dibaj Clinic within three days of symptom onset and had a positive RT-PCR test, SPo2 > 92%, and normal chest CT on their initial visit. Patients without respiratory distress, who had not received any COVID-19 vaccine, were included in this research.
Exclusion criteriaWe did not include patients who did not meet the following criteria: those undergoing other therapies, patients with BMI < 18.5 and BMI > 25, patients with underlying conditions such as pregnant or lactating patients, patients receiving nitroglycerin, those who showed evidence of lung involvement (oxygen levels >92%, persistent symptoms of shortness of breath and chest pain, and evidence of lung involvement seen in CT scans) requiring hospitalization or referral to a hospital, as recommended by the infectious diseases specialist at the hospital, and those who have a history of allergy or anaphylactic shock to N-acetylcysteine or Bromhexine or have experienced side effects while using them.
Data analysis methodWe used the independent t-test to compare quantitative variables, the chi-square test to compare qualitative variables, and ANOVA test to compare the means of three or more groups. If necessary, we analyzed the results using a Poisson regression model. All statistical analyses were performed at a 95% confidence level using Stata software, version 16.
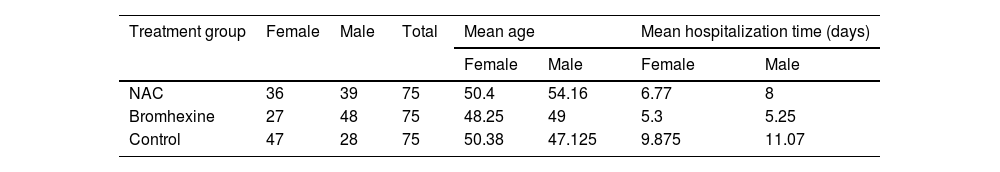
ResultsThe present study aimed to investigate the efficacy of N-acetylcysteine (NAC) and Bromhexine in treating COVID-19 positive patients. The study included a total of 225 outpatients who were referred to Dibaj Medical Center after being tested positive for COVID-19 through PCR. The patients were randomly assigned to one of three groups: 75 patients received NAC, 75 patients were treated with Bromhexine, and 75 patients served as a control group and received no medication. Among the total sample, 110 individuals (48.9%) were female and 115 individuals (51.1%) were male. The mean age of the patients was 45.31 ± 14.884 years with a range of 18 to 80 years. Among the 225 patients, 152 (67.6%) had no underlying diseases, 29 (12.9%) had hypertension, 21 (9.3%) had cardiovascular disease, 15 (6.7%) had diabetes, and 4 individuals (1.8%) had cancer. Additionally, 4 individuals (1.8%) had other diseases.
Out of 225 patients, 38 (16.88%) were hospitalized, while 187 (83.11%) had no history of hospitalization within a month. In the NAC group, 11 out of 75 patients (14.66%) were admitted to the hospital, while the remaining 64 patients (85.33%) recovered at home without hospitalization. In the Bromhexine group, 6 out of 75 patients (8%) were hospitalized, and the remaining 69 patients (92%) were treated at home without requiring hospitalization. In the control group, 21 out of 75 patients (28%) were admitted to the hospital, and the remaining 54 patients (72%) were treated at home without hospitalization.Overall, the average age of hospitalized patients was 55.15 years, ranging from 24 to 80. Among the 38 patients admitted to the hospital, 58.8% were over 60 years old, while 45.31% were under 60 years old. (Table 1).
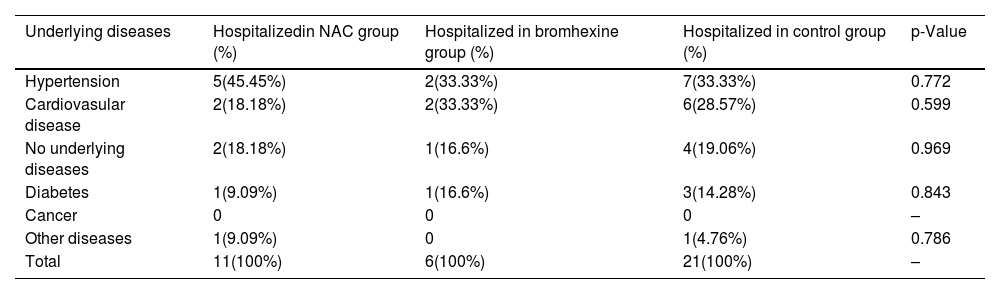
The association between hospitalization rates and the underlying diseases of patients is as follows: out of 38 hospitalized patients, 14 (36.82%) had hypertension, 10 (26.31%) had cardiovascular disease, 7 (18.42%) had no underlying diseases, 5 (13.15%) had diabetes, and 2 (5.26%) had other diseases.Moreover, the comparison of hospitalization rates for different underlying diseases among the NAC, Bromhexine, and Control groups did not reveal any statistically significant differences (p-value > 0.05) (see Table 2).
Comparison of hospitalization rates for different underlying diseases among NAC, Bromhexine, and control groups: chi-square analysis.
| Underlying diseases | Hospitalizedin NAC group (%) | Hospitalized in bromhexine group (%) | Hospitalized in control group (%) | p-Value |
|---|---|---|---|---|
| Hypertension | 5(45.45%) | 2(33.33%) | 7(33.33%) | 0.772 |
| Cardiovasular disease | 2(18.18%) | 2(33.33%) | 6(28.57%) | 0.599 |
| No underlying diseases | 2(18.18%) | 1(16.6%) | 4(19.06%) | 0.969 |
| Diabetes | 1(9.09%) | 1(16.6%) | 3(14.28%) | 0.843 |
| Cancer | 0 | 0 | 0 | – |
| Other diseases | 1(9.09%) | 0 | 1(4.76%) | 0.786 |
| Total | 11(100%) | 6(100%) | 21(100%) | – |
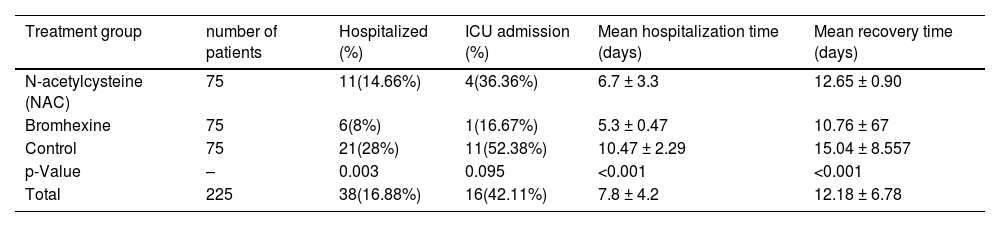
In the NAC group, 4(4.4%) out of 11 hospitalized patients required ICU admission, whereas 1(2.4%) out of 6 hospitalized patients in the Bromhexine group required ICU admission. Among the control group, 11(10.2%) out of 21 hospitalized patients required ICU admission. The mean hospitalization time was 5.8, 5, and 9.63 days for groups A, B, and C, respectively. In the Bromhexine group, the mean hospitalization time was 5.25 days in males and 5.3 days in females. In the NAC group, the mean hospitalization time was 5.4 days in females and 8 days in males. In the control group, the mean hospitalization time was 11.07 days in males and 9.875 days in females respectively and the difference between these three groups is statistically significant (p = 0.766).In general, the average recovery time of the patients (standard deviation) from the symptom appearance to the end of the symptoms was 12.18 (6.78) days, with respective minimum and the maximum recovery periods of 3 and 40 days after the emergence of symptoms. The mean duration of complete recovery of symptoms in NAC and Bromhexine groups was 12.65 ± 0.90 and 10.76 ± 0.64 (p = 0.0935), respectively, showing no statistically significant difference but in control group was 15.04 ± 8.557 (p = 0.0001) showing statistically significant difference (Table 3).
This table presents the outcomes of three treatment groups (N-acetylcysteine, Bromhexine, Control) in this study. The data includes hospitalization and ICU admission percentages, as well as mean hospitalization and recovery times. Statistical analyses include the Chi-squared test for hospitalization and ICU admission percentages, and One-Way ANOVA for mean hospitalization and recovery times, followed by post hoc tests for significant differences.
| Treatment group | number of patients | Hospitalized (%) | ICU admission (%) | Mean hospitalization time (days) | Mean recovery time (days) |
|---|---|---|---|---|---|
| N-acetylcysteine (NAC) | 75 | 11(14.66%) | 4(36.36%) | 6.7 ± 3.3 | 12.65 ± 0.90 |
| Bromhexine | 75 | 6(8%) | 1(16.67%) | 5.3 ± 0.47 | 10.76 ± 67 |
| Control | 75 | 21(28%) | 11(52.38%) | 10.47 ± 2.29 | 15.04 ± 8.557 |
| p-Value | – | 0.003 | 0.095 | <0.001 | <0.001 |
| Total | 225 | 38(16.88%) | 16(42.11%) | 7.8 ± 4.2 | 12.18 ± 6.78 |
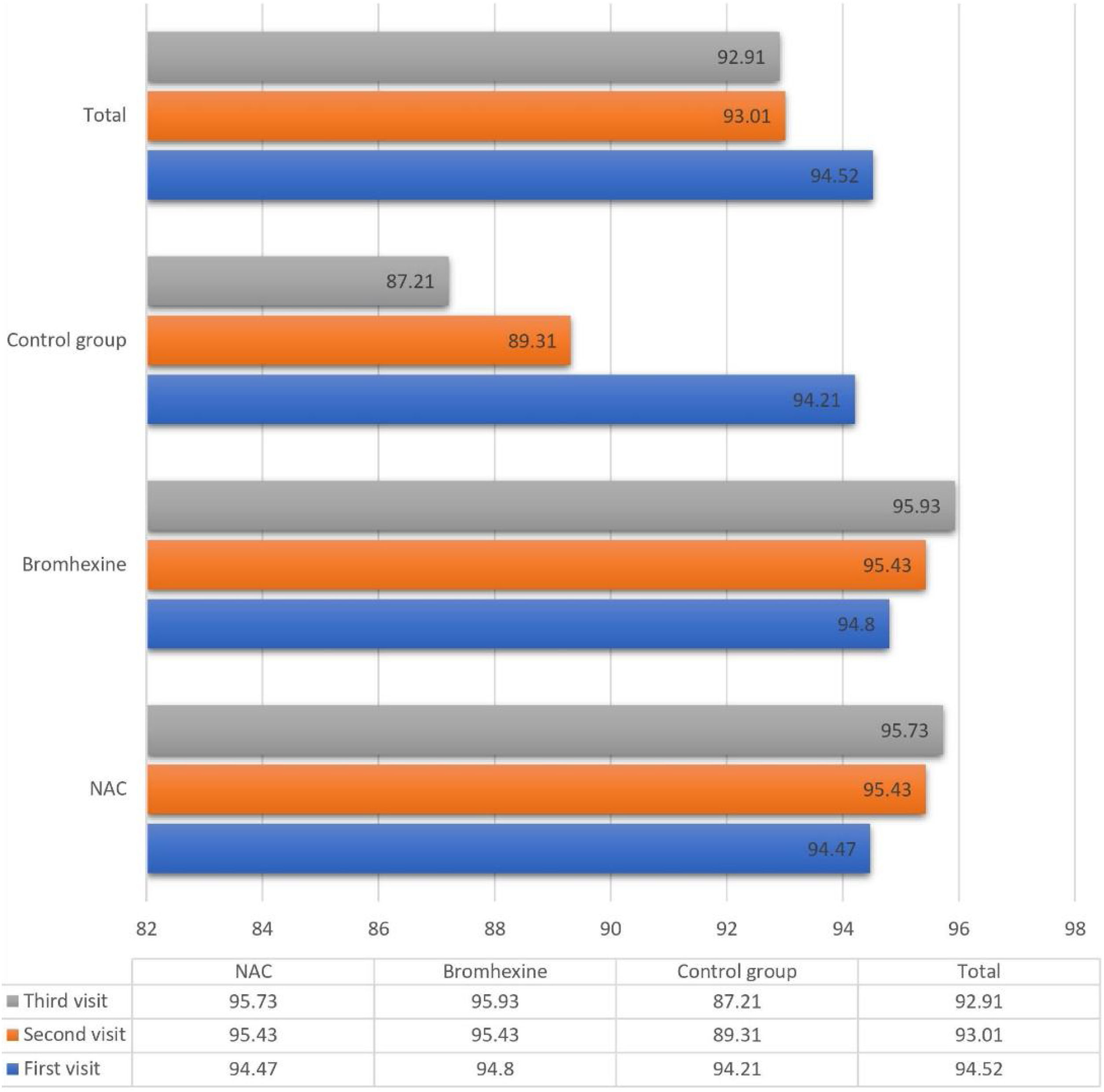
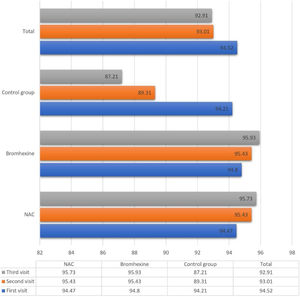
The average oxygen saturation levels across the three study groups exhibited nuanced variations. On the initial visit, all groups manifested a comparable mean saturation of 94.52 ± 2.502%. Subsequent assessments on the seventh and fourteenth days demonstrated a decline to 93.01 ± 6.862% and 92.91 ± 12.55%, respectively.
Upon closer inspection within the NAC group, oxygen saturation displayed distinct trends across sequential visits: 94.47%, 95.43%, and 95.73% on the first, second, and third visits, respectively. Analogously, the Bromhexine group showcased levels of 94.80%, 95.43%, and 95.93% during the corresponding visits. Conversely, in the untreated control group, oxygen saturation declined from 94.21% to 89.31% and further to 87.21% during the first, second, and third visits, respectively.
Application of the ANOVA test yielded noteworthy findings in oxygen saturation levels:
On the first day, no statistically significant divergence existed in the average oxygen concentration across the three groups (p = 0.393).
By the seventh day, a significant distinction emerged (p < 0.001), with NAC and Bromhexine groups evidencing notably higher concentrations compared to the control group.
A similar pattern persisted on the fourteenth day, signifying a significant disparity (p < 0.001) in oxygen concentration, favoring NAC and Bromhexine groups.
Further scrutiny of oxygen saturation levels elucidated compelling temporal dynamics:
Between the first and second visits, NAC and Bromhexine groups exhibited a statistically significant increase, while the control group experienced a decrease (p < 0.001).
Analogously, between the second and third visits, NAC and Bromhexine groups displayed a rise in saturation levels, while the control group exhibited a decline (p < 0.001) (Fig. 1).
Notably, mortality rates were nil in Groups A (NAC) and B (Bromhexine), contrasting with seven deaths (9.33%) recorded in Group C (control). A noteworthy 78% of patients reported no post-medication complications, with mild complications reported by 33 individuals, predominantly manageable without intervention. In-depth analysis within the NAC and Bromhexine groups elucidated specific complications, including blood pressure reduction and stomach pain in NAC, and drowsiness in Bromhexine, with no reported study discontinuations due to complications. Remarkably, no patients withdrew from the study owing to adverse effects.
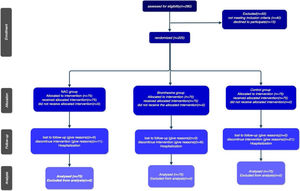
The study's procedural chronology, depicted through a Consort diagram, delineates patient segmentation based on subsequent follow-up periods until the culmination of outcomes )Fig. 2).
DiscussionIn this study, the efficacy of N-acetylcysteine (NAC) and Bromhexine in treating COVID-19-positive patients were investigated, and the results were compared with a control group that received no medication. The study included a total of 225 patients, randomly assigned to three groups. The study results indicate that the hospitalization rate was lower in both the NAC and Bromhexine groups compared to the control group, and the rate of admission in icu was reduced. Although the difference was not statistically significant, the mean hospitalization time was shorter in the treatment groups compared to the control group.
Furthermore, our study, along with the research carried out by Izquierdo JL et al., indicates that N-acetylcysteine (NAC) exhibits promising outcomes in mitigating patient mortality and reducing intensive care unit admission in the treatment of COVID-19.18 The study conduct by Milara et al. examined the activation of the body's defense system known as the inflammasome in severely ill COVID-19 patients, along with the mechanistic impact of N-acetylcysteine (NAC) on this inflammatory process. Small airway samples were collected from patients, and genetic and protein analyses were conducted. The inflammasome demonstrated heightened activity in severe COVID-19 cases, with even greater activation observed in the intense variant. The findings indicated that NAC exhibited the potential to mitigate inflammasome activity when exposed to the virus in a laboratory environment. This suggests a prospective therapeutic application of NAC in the treatment of COVID-19 patients.19 Also, in a study conducted by Galindo-Andugar et al. on 378 patients, 52.6% of whom received NAC treatment, they concluded that NAC treatment was identified as a protective factor against mortality, which It was the same as the result of our study.22 In the other study conducted by Alam MS et al., The systematic review and meta-analysis assessed the therapeutic impact of N-acetylcysteine (NAC), an antioxidant, on COVID-19. Analyzing eight studies with 20,503 participants, it found that NAC treatment was associated with lower mortality compared to the placebo group (RR: 0.65). Additionally, NAC resulted in significant reductions in C-reactive protein (CRP) and D-dimer levels, and an increase in the oxygenation marker PaO2/FiO2 ratio. Despite the limited number of studies, the findings suggest a positive effect of NAC on COVID-19 outcomes, emphasizing decreased mortality and improvements in inflammatory markers and oxygenation.23 Similarly, the study conducted by Taher et al. supports the beneficial effect of NAC in treating Covid-19 patients.24 Moreover, the investigation conducted by Du Preez HN et al. produced results consistent with our study, demonstrating positive outcomes with the use of NAC.25 Although these studies provide encouraging findings, it is important to continue exploring and validating the efficacy of NAC as a treatment for Covid-19.
In a study conducted by Ansarin et al., which is similar to our research, it was concluded that Bromhexine has helpful significant improvements in clinical outcomes and even mortality rates.20 In another study written by Tolouian et al., the use of Bromhexine was found to aid in the recovery of COVID-19 patients, but unlike our study, it did not have an effect on mortality.26
In our study, we observed that in all groups, the hospitalization and mortality rates were higher in men and among the elderly population. These findings are consistent with the study conducted by Hesni et al. in Kermanshah17 Furthermore, the average recovery time of the patients in the NAC and Bromhexine groups was comparable, and both groups had a shorter recovery time compared to the control group. However, the difference in recovery time was not statistically significant between the treatment groups. In a study written by Tolouian et al., the use of Bromhexine was found to be helpful in decreasing the duration of hospitalization.26
In terms of oxygen saturation levels, the results showed that the average oxygen saturation in the treatment groups was higher compared to the control group, particularly on the seventh day of the disease. The average oxygen saturation levels were also comparable between the NAC and Bromhexine groups.
The mortality rate among patients in groups A and B who received the medication was zero, which aligns with findings from previous studies, such as Ansarin's research.20 However, the mortality rate in the control group was significantly higher compared to the figures reported by Johns Hopkins Coronavirus Resource Center.21
One possible explanation for this discrepancy is that we only included patients who had not received the COVID-19 vaccine, which is a known risk factor.18 In our study, we conducted a comparison between the association of underlying diseases and Covid-19. We found that hypertension patients had the highest death rate, accounting for approximately 49% of the total deaths. This finding aligns with a study conducted by Javanmardi et al., where they reported a mortality rate ranging from 55% to 37% among hypertension patients, further supporting our observations. Furthermore, we examined the mortality rate of patients with cardiovascular disease in our study, which was recorded at 28.6%. This result closely resembles the findings reported by Javanmardi et al., who noted a similar mortality rate ranging from 16% to 27% among heart patients. One possible explanation for the disparity in mortality rates between two groups (Group A and Group B) and Group C could be attributed to the fact that prompt treatment and early detection can significantly contribute to preventing patient mortality.27
Moreover, the results of this study indicate that NAC (N-acetylcysteine) and Bromhexine may be effective in treating COVID-19-positive patients. These treatments were associated with a lower hospitalization rate, shorter hospitalization time, and improved oxygen saturation levels. In summary, early detection and prompt treatment play a crucial role in reducing mortality rates. Additionally, preliminary evidence suggests the potential effectiveness of NAC and Bromhexine in treating COVID-19 patients. However, it is important to note that further studies with larger sample sizes and longer follow-up periods are needed to confirm these findings.
ConclusionsThe present study was conducted with the aim of investigating the effectiveness of N-acetylcysteine (NAC) and bromhexine in the treatment of patients with positive COVID-19. The findings of the study show that both NAC and Bromhexine groups showed a lower hospitalization rate than the control group. In addition, a lower percentage of patients required ICU admission in the treatment groups. Although the use of two drugs, Bromhexine and NAC, did not have statistically significant changes in the hospitalization rate of patients with underlying disease and patients without underlying disease, but it was very noticeable in the mortality rate of people. However, underlying diseases are still the biggest risk factors. The average hospitalization time in the treatment groups was also shorter than in the control group. In addition, the mean time of improvement of symptoms was shorter in the NAC and bromhexine group than in the control group. However, there was no statistically significant difference between NAC and Bromhexine in terms of length of hospital stay or time to symptom resolution.
In conclusion, the results of this study indicate that NAC and bromhexine may be effective in the treatment of patients with positive covid-19, with a lower hospitalization rate, shorter hospitalization, faster recovery time, and reduced mortality compared to the control group. Nevertheless, it is necessary to continue to explore and validate the efficacy of NAC and bromhexine as a treatment for Covid-19, as these findings provide encouraging but preliminary evidence.
LimitationsInitially, the study was conducted in a single clinic in Iran with a specific population, and the findings may not be generalizable to other populations or settings. Second, the study excluded patients with underlying medical conditions, those requiring hospitalization, and those who had received the COVID-19 vaccine, limiting the generalizability of the findings to these specific patient groups in the end, numerous risk factors for COVID-19, including underlying diseases, advanced age, obesity, and vaccination, among others, have been identified in prior studies. This study focused on some of these factors, while others couldn't be investigated due to conflicts with the cultural context of my country.
Ethics approval and consent to participateThis study was approved by the Research Ethics Committees of Hamadan University of Medical Science, with the ethics code IR.UMSHA.REC.1400.957. Additionally, it was approved by the judges of the International Center for Registration of Clinical Trials of Iran, a member of the international centers approved by the World Health Organization, with the code IRCT20220302054167N1with Registration date: 2022-04-29. Informed consent was obtained from all participating patients or their legal guardians in this study after fully explaining the study methods, drug side effects, and other necessary matters. Prior to participation, all patients were screened for drug allergy history. The study was fully explained to the patients, and all their questions and doubts were addressed. It was also emphasized to the patients that they could withdraw from the study at any time without affecting their treatment. All patients were assured that their personal information would remain confidential and that only collective information from the entire study would be available. Finally, all methods were carried out in accordance with relevant guidelines and regulations. Therefore, conducting the research was in accordance with the ethical principles of the Declaration of Helsinki.
Consent for publicationNot applicable.
Availability of data and materialsAll data generated or analyzed during this study are included in this published article.
Conflicts of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
FundingThis study was supported by internal funding. All authors read and approved the final manuscript.